All published articles of this journal are available on ScienceDirect.
Atrophosclerodermic Manifestations of Lyme Borreliosis
Abstract
This review summarizes the literature on scleratrophic skin lesions as a manifestation of a Borrelia infection. An association of morphea with Lyme borreliosis was mainly reported from Middle-European Countries, Japan and South America. B. afzelii has been identified predominantly from the chronic skin lesions of acrodermatitis chronica atrophicans (ACA) and has been cultivated from morphea lesions in isolated cases. Scleratrophic skin lesions like morphea, lichen sclerosus et atrophicus (LSA) and anetoderma have been observed in coexistence with ACA. Since all these diseases show clinical and histological similarities, they might have a common origin. The laboratory results that point to a borrelial origin of these diseases, however, are contradictory. Antibodies against B. burgdorferi were detected in up to 50% of patients. Borrelia DNA was shown in up to 33% of morphea and 50% of LSA patients. Borrelia were visualized on histological slides by polyclonal antibodies in up to 69% of morphea and 63% of LSA patients. In other reports no evidence of Borrelia – associated morphea or LSA has been reported. For anetoderma, single case reports showed positive Borrelia serology and/or PCR and a response to antibiotic treatment. The response of scleratrophic skin lesions to antibiotic treatment varies and can be seen in patients with or without a proven association to a Borrelia infection. This suggests that scleratrophic diseases might be of heterogeneous origin, but a Borrelia infection could be one cause of these dermatoses.
INTRODUCTION
Lyme borreliosis (LB) is a world-wide infectious disease with classical clinical manifestations in the skin, namely erythema migrans (EM) and borrelia lymphocytoma in the early and acrodermatitis chronica atrophicans (ACA) in the late stage.
In 1985 three research groups first suggested that morphea (localized scleroderma) could be another manifestation of a Borrelia burgdorferi infection [1-3]. The detailed development of research work in morphea and lichen sclerosus et atrophicus (LSA) and the association with LB was reviewed by Weide et al. in 2000 [4]. A complete summary of findings on the role of B. burgdorferi using serology, culture, PCR, immunohistochemistry, silver staining, lymphocyte stimulation test and treatment response has been published. The findings were also classified according to the respective geographic areas and countries. In 2000 and 2010, further reviews were published addressing the detection of Borrelia DNA by PCR-based methods in morphea and lichen sclerosus et atrophicus (LSA) [5, 6].
FIBROTIC CHANGES IN ACA, COEXISTENCE WITH MORPHEA AND LICHEN SCLEROSUS ET ATROPHICUS
Asbrink was one of the first to observe sclerotic changes in ACA in the context of a Borrelia infection [7]. Juxtaarticular nodules occur in 10-25% of ACA patients (Fig. 1). These can arise after the onset of ACA [8], or also as an isolated manifestation of LB (authors’ observation). There have been a number of reports of localized or widespread fibrotic lesions in B. burgdorferi infection, as well as coexistence of ACA and morphea [8-14] (Fig. 2). Among 10 seropositive morphea patients, Büchner et al. described 2 patients with a history of EM, 1 with coexisting ACA, and 2 with LSA [15]. In 1994 Trevisan et al. reviewed the association of B. burgdorferi and localized scleroderma [16].
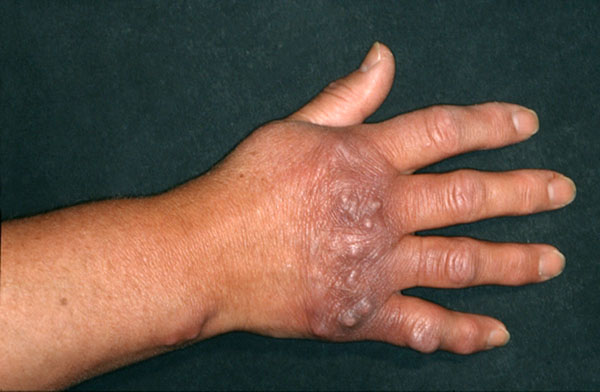
Fibrous nodules in acrodermatitis chronica atrophicans.
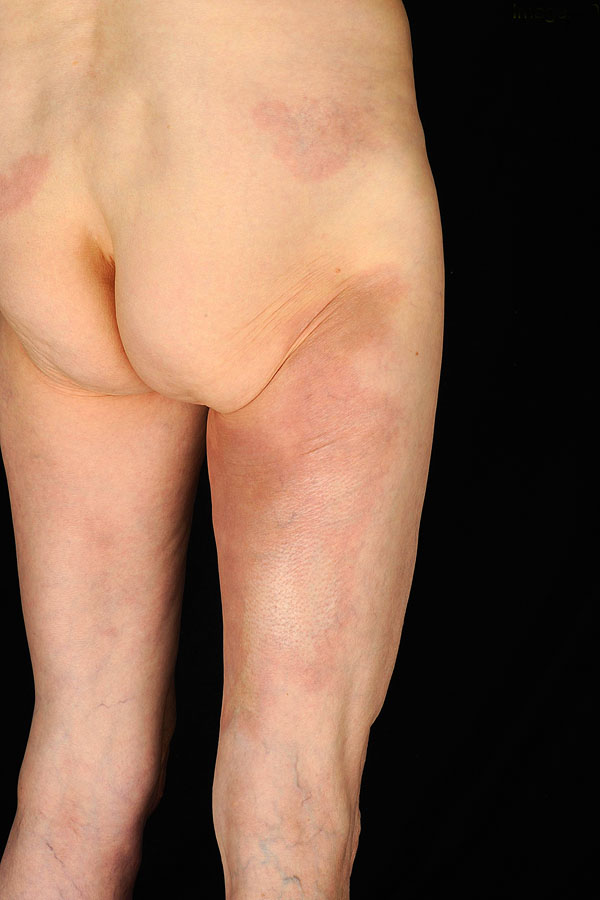
Acrodermatitis chronica atrophicans with pseudoscleroderma and erythematous patches on the buttock presenting early morphea.
ACA was described in association with genital and extragenital LSA, tubulo-interstitial nephritis and spirochetes in urine that were seen one year after a tick bite [17]. ACA, LSA and nephritis regressed after antibiotic therapy.
MORPHOLOGICAL FINDINGS IN MORPHEA, LSA AND ACA
Clinically, early morphea cannot be distinguished from EM, and persisting EM cannot be distinguished from early morphea in terms of clinical or histological features in some patients. Morphea can mimic ACA as well, with a livid discoloration (Fig. 3). Whereas ACA involves acral body sites with lower temperatures, is located on the extensor surfaces of extremities and not sharply demarcated (Fig. 4), morphea is sharply demarcated, appears as single or multiple plaques that can be linear, and/or deep (including fasciitis) with possible confluence of lesions, affects any age and body site, and enlarges at the periphery of the lesions with the typical lilac ring.
Morphea is a disease of heterogeneous origin occurring after vaccination, trauma, or insect bites; it can also be congenital [18]. Histologically, the early phase is associated with edematous swelling of collagen fibers, perivascular and interstitial infiltration of lymphocytes, monocytes, histiocytes, eosinophils and plasma cells, followed by collagen overproduction or atrophy [19]. Serup divided the connective tissue changes in morphea into fibril degradation and myxedematous, cell infiltration and fibrotic stages [20]. Upon electron microscopy, collagen changes include loss of their transverse striation, disintegration of fibers, elastoid transformation and complete collagen destruction [21].
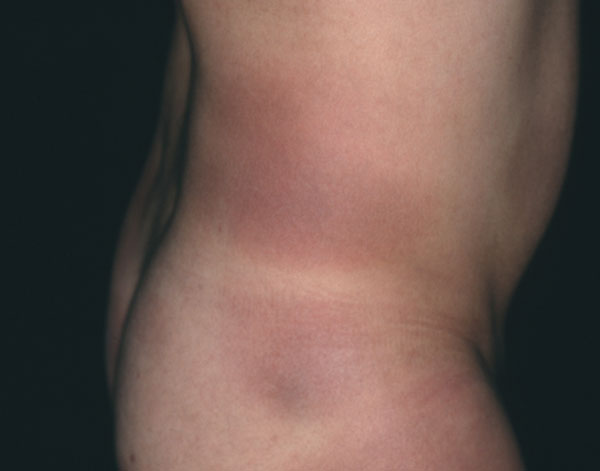
Livid patches of morphea on the trunk. Culture of a skin biopsy was positive. B. afzelii was identified by PCR-RLFP analysis [111].
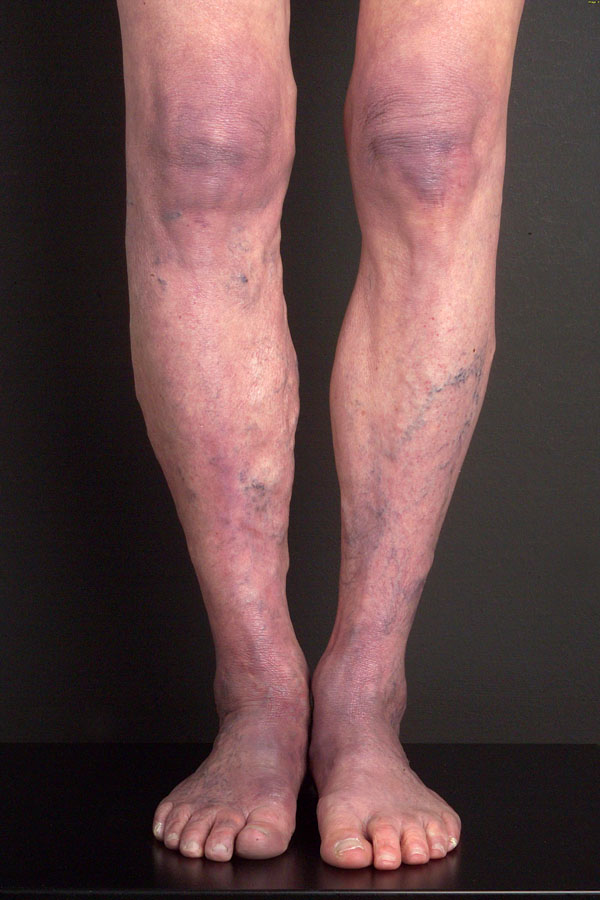
Acrodermatitis with atrophic skin changes.
Histologically, ACA shows both a band-like subepidermal and a perineural cell infiltrate, atrophy of collagen with edematous or thinned collagen fibers, and fibrosis. There are no important differences between the lesional skin of ACA, fibrous nodules, ulnar bands or sclerodermatous changes [22]. Electron microscopy shows that elastic fibers are destroyed and collagen fibers are present with peripheral amorphic material in ACA [23], showing similarities to the described changes in morphea [21].
Comparing the histological features and the inflammatory infiltrate in 19 ACA and 40 morphea biopsies, histological differentiation from ACA was not possible in 17% of morphea cases regardless of positive or negative B. burgdorferi serology [24]. Both diseases display basal cytolysis of keratinocytes. In ACA the inflammatory infiltrate shows a lichenoid, in morphea a perivascular pattern. Hypertrophic basophilic elastic tissue is seen in ACA, whereas in morphea the elastic tissue is preserved and polarizing. The histological pattern of morphea could not be associated with a particular clinical type of morphea or with positive B. burgdorferi serology with exception of atrophodermia Pasini-Pierini, where an association with a Borrelia infection could not be detected in that study [25]. Thus, a Borrelia etiology cannot be established from a morphological pattern in morphea.
LSA also shows an atrophic epidermis with basal layer vacuolization [26]. The superficial dermis is edematous and hyalinized with disappearance of elastic fibers, although there is an increase in elastic fibers in the mid and lower dermis. Collagen degeneration and regeneration were observed ultrastructurally in the superficial dermis with higher amounts of ground substance [27]. Collagen fibers were also observed to be phagocytized by fibroblasts [28]. Coexistence of LSA with morphea was observed in 27 of 472 (5.7%) patients [29].
In summary, ACA, morphea and LSA share histopathological similarities like vacuolization of basal keratinocytes and loss of collagen; ACA and morphea the fibrosing tissue reaction; and ACA and LSA loss and proliferation of elastic fibers.
METHODS
A PubMed search was performed in December 2014 with the following key words: 1) “morphea, borreliosis”: 97 papers were published in the last 30 years with original publications, case series, case reports and reviews showing an association with a B. burgdorferi infection or no evidence. The majority of these publications are analyzed in this review. 2) “anetoderma, borreliosis” produced 6 papers, 4 of them case reports and 2 of them reviews pointing out that sclerotic and atrophic lesions of morphea can be observed in the spectrum of LB. 3) “atrophy, borreliosis, skin”: 26 papers were published, 14 of them about ACA, 6 about scleratrophic findings, and 3 about Parry-Romberg syndrome; there were also 3 reviews and other miscellaneous papers.
Table 1 lists research papers on morphea and LSA in relation to a Borrelia infection that have been published since 2000; Table 2 lists publications about anetoderma; and Table 3 lists clinical cases of sclerotic skin lesions that were treated with antibiotics.
Reports on the association of B. burgdorferi with morphea and lichen sclerosus et atrophicus since 2000.
| Author, year, country | No. of patients/controls | Serology % pos. (pos./ no. of patients) | PCR % pos (pos./ no. of patients)/primer | Immuno-histochemistry % pos. (pos./ no. of patients)/used antibodies |
|---|---|---|---|---|
| Özkan S [5] 2000 Turkey |
10 morphea 12 LSA10 controls | n.d. | 33% (3/10)50% (6/12) nested PCR, flagellin primer | n.d |
| Svecova D [38] 2000 Slovakia |
32 morphea7 LSA131 controls55 LB patients | 34.4% (11/32) 71.4% (5/7) 33.6% (44/131)100% (55/55) | n.d. | n.d. |
| Wojas-Pelc A [39] 2002 Poland |
50 morphea | 28.5% ANA 18% |
n.d. | n.d. |
| Espinoza-Leon F [55] 2006 Venezuela |
21 morphea21 controls | 14% (3/21) 4.7% (1/21) positive by ELISA not confirmed by IB | n.d. | n.d. |
| Sommer A [54] 2006 Germany (Bochum) |
12 hemiatrophia faciei | 3 positive ELISA not confirmed by IB | n.d. | n.d. |
| Eisendle K [59] 2007 Austria |
122 morphea10 controls with pos PCR 58 controls with neg PCR | n.d. | 1/30 nested PCR B.burgdorferi 23S rRNA | 69% (84/122)100% (10/10)0% (0/0) polyclonal antibodies |
| Eisendle K [110] 2008 Austria |
60 LSA68 controls (classic LB) | n.d. | n.d. | 63% (38/60)90% (61/68)/polyclonal antibodies, FFM |
| Prinz JC [40] 2009 Germany (Bavaria) Hungary |
90 morphea | 22% (20/90) ANA 47% (42/90) | n.d. | n.d. |
| Zollinger T [6] 2010 Switzerland |
49 morphea15 LSA 48 controls (granuloma anulare) | n.d. | 2% (1/49)7% (1/15)2% (1/48) /nested PCR; sequence analysis: B. burgdorferi sensu stricto | n.d. |
| Espinoza-Leon F [69] 2010 Venezuela |
21 morphea | n.d. | 0 % (0/21) | n.d. |
| Santos M [60] 2011 Amazonic region |
15 morphea, atrophodermia Pasini-Pierini, LSA | n.d. | n.d. | 26.6% (4/15) / FFM |
EVIDENCE OF B. BURGDORFERI INFECTION IN MORPHEA (Table 1)
Serology
In the review of Weide et al. covering 1985 to 2000, a total of 138 among 609 morphea cases with positive anti-B. burgdorferi antibodies were reported from Austria, Germany, Italy, Poland, Slovakia, and Switzerland [4, 25, 30-38]. Table 1 lists the publications since 2000 that show a possible association of morphea with B. burgdorferi infection. Anti-B. burgdorferi antibodies were reported in 22 - 34.4% of morphea patients [38-40]. Wojas-Pelc et al. showed positive B. burgdorferi serology in 28.5% and positive ANA in 18% [39]. Prinz et al. observed that seropositive patients with early-onset morphea had high ANA titres [40].
Negative or negligible serologic findings were reported from Denmark, England, Finland, France, Japan, Netherlands, Spain, Venezuela, and USA [41-55].
Morphological Detection of B. burgdorferi by Immunohistochemistry or Silver Staining
In 1987 Aberer et al. detected Borrelia in 7/21 biopsies of morphea patients by immunohistochemistry. Diluted serum from an ACA patient was used and Borrelia were visualized by high-power resolution videomicroscopy [56]. In a further study Borrelia were detected in 25% of morphea, EM and ACA biopsies [57]. Borrelia could also be visualized by silver staining in 10/25 morphea patients in Puerto Rico [58], and by a polyclonal antibody in 84/122 morphea patients using focus floating microscopy (FFM) [59]. Only 1 of these 30 patients was positive by PCR. With FFM, Borrelia were detected in morphea in 3/15 patients in Brazil, LSA and atrophodermia [60] and in a case of a boy with early-onset morphea with tight skin, whereby PCR was negative [61]. Immunohistochemistry was negative in 14 morphea biopsies [47] as well as Elias-Bosma staining in 13 morphea patients [62].
PCR
In 1993 Schempp et al. showed B. burgdorferi-specific DNA with flagellin primers of the 16s-RNA gene in lesional skin of patients with morphea [48]. In a follow-up study, the results of the positive PCR were compared, since patients` biopsies were derived from 3 different countries. Whereas some of the biopsies of German and Japanese patients were positive, biopsies from the USA were negative [63]. PCR was positive in seropositive and seronegative Italian morphea patients [37]. Nested PCR was performed for Turkish morphea patients and 3/10 of them were positive [5]. Borrelia DNA was detected in different sclerodermiform skin lesions [64, 65]. Amplification of DNA sequences of B. burgdorferi by nested PCR, and proven by sequencing was only positive in 1/49 morphea patients in Switzerland [6]. No Borrelia-specific DNA has been detected in morphea lesions in several other studies from Finland, Netherlands, Spain, USA [49-51, 53, 66-69] and also in one study from Germany [33].
Culture
B. burgdorferi from skin biopsies of morphea was only cultured for a minority of patients, with low outcome [56, 70] (Fig. 3). B. afzelii was cultured from a seronegative patient with widespread morphea identified by SDS polyacrylamide-electrophoresesis of outer surface proteins [71]. Further studies culturing B. burgdorferi from morphea were negative [47, 49, 53].
Lymphocyte Transformation Test (LTT)
In 1993 Büchner et al. found significantly elevated lymphoproliferative responses in 10 seropositive morphea patients [15]. In a further study lymphoproliferative responses were measured in 45 patients with morphea compared to 21 healthy seronegative controls and patients with EM, lymphadenosis benigna cutis and ACA. Stimulation indices in morphea patients did not differ significantly from the normal population [34].
LTT was positive in a single seronegative morphea case with positive PCR by flagellin primers and immunohistochemical detection of B. burgdorferi in the skin [72]. Breier et al. reported elevated mean stimulation indices in 11/39 (28%) of seropositive and seronegative morphea patients; these were nearly as high as in patients with EM compared to ACA, seropositive LB patients and healthy controls [36].
Eosinophilic Fasciitis/Shulman Syndrome/Parry-Romberg Syndrome
Positive Borrelia serology and an association with LB were often reported in eosinophilic fasciitis/Shulman syndrome (Fig. 5) [73-78], with detection of a positive flagellin gene sequence by PCR in one patient [79]. In 4 cases of borrelial fasciitis, spirochetal organisms were identified by Dieterle and Steiner silver stains; one patient was positive with a rabbit polyclonal antibody; 2/4 patients were seropositive [80].
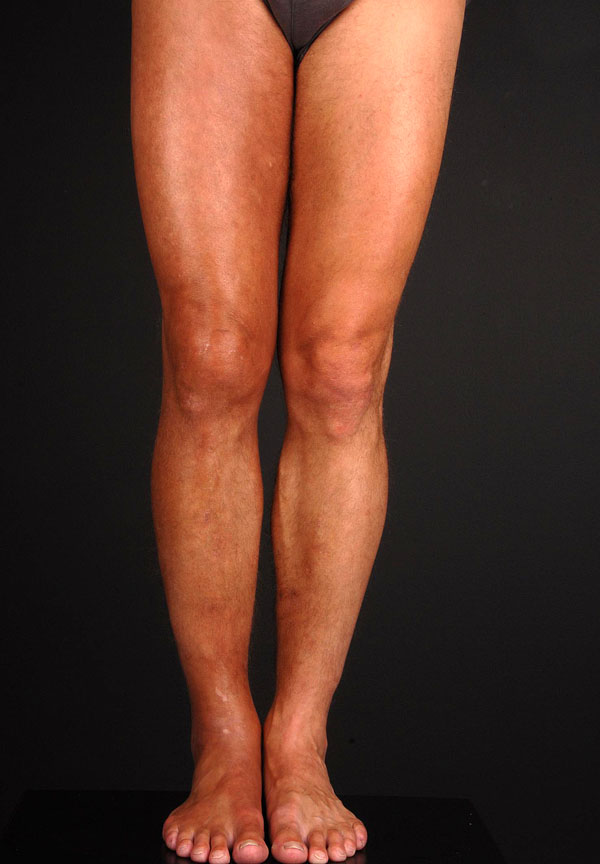
Eosinophilic fasciitis arising in the context of Lyme arthritis of the knee on the same extremity.
No clear association with Borrelia infection was shown in Parry-Romberg syndrome [81-83]. In a retrospective study, 2 of the 12 patients with hemiatrophy were seropositive by ELISA that was not confirmed by IB [54]. In one Italian patient with linear scleroderma “en coup de sabre” with facial atrophy, antibodies against B. burgdorferi were positive [84].
EVIDENCE OF B. BURGDORFERI INFECTION IN LICHEN SCLEROSUS ET ATROPHICUS (TABLE 1)
There are only limited studies on the determination of anti-B. burgdorferi antibodies in LSA. Svecova et al. from Slovakia found 5/7 seropositive LSA patients [38], and Aberer et al. 2/13 seropositive LSA patients [56].
Immunohistochemistry/Silver Staining
In 6/13 patients with genital and extragenital LSA, Borrelia could be detected by immunohistology after staining with a 1:20 diluted serum from a patient with high antibody titers against B. burgdorferi visualized by videomicroscopy [56]. Using FFM, Eisendle et al. detected Borrelia species in 38 of 60 cases (63%) of lichen sclerosus and in 61 of 68 (90%) positive controls of classic borreliosis, but Borrelia species were absent in all negative controls. Borrelia species were detected significantly more often in early inflammatory-rich (31 of 39 80%) than in late inflammatory-poor (7 of 21 33.3%) cases (P = .001). Polymerase chain reaction findings were positive in 25 of 68 positive controls (37%) and negative in all 11 cases of lichen sclerosus and all 15 negative controls [85].
PCR in Skin Biopsies
Özkan et al. demonstrated the presence of B. burgdorferi DNA in 6/12 patients with LSA by a nested PCR using European flagellin gene primer sets [5]. In contrast, negative molecular biological findings were reported in LSA biopsies by PCR using OspA or flagellin or clone 2H1 primers [53, 66, 67].
Zollinger et al. performed a PCR-based study and reviewed the literature. The authors could only detect Borrelia specific DNA in 1/15 (6.6%) LSA patients (1/49 morphea, 1/48 granuloma annulare) and assumed that B. burgdorferi does not play a significant role in these diseases [6].
Urine PCR
Amplification of Borrelia DNA in the urine was positive by flagellin primer in 13/19 patients with LSA (none in 23 healthy controls and 49 patients with various dermatologic diseases except in granuloma anulare in 8/13 urines [86].
Culture
Culture for B. burgdorferi was negative in 1 investigated LSA patient [53].
ATROPHIC LESIONS IN LYME BORRELIOSIS (Table 2)
Anetoderma is defined by a decreased amount of dermal elastic tissue and a variable amount of inflammatory infiltrate [87]. A patient with multiple oval skin colored macules with a wrinkled surface is reported. PCR analysis for the flagellin gene and genotyping were positive for B. afzelii. Strunk et al. reported another patient with positive IgM antibodies to B. burgdorferi and arrested progression of anetoderma after doxycycline [88].
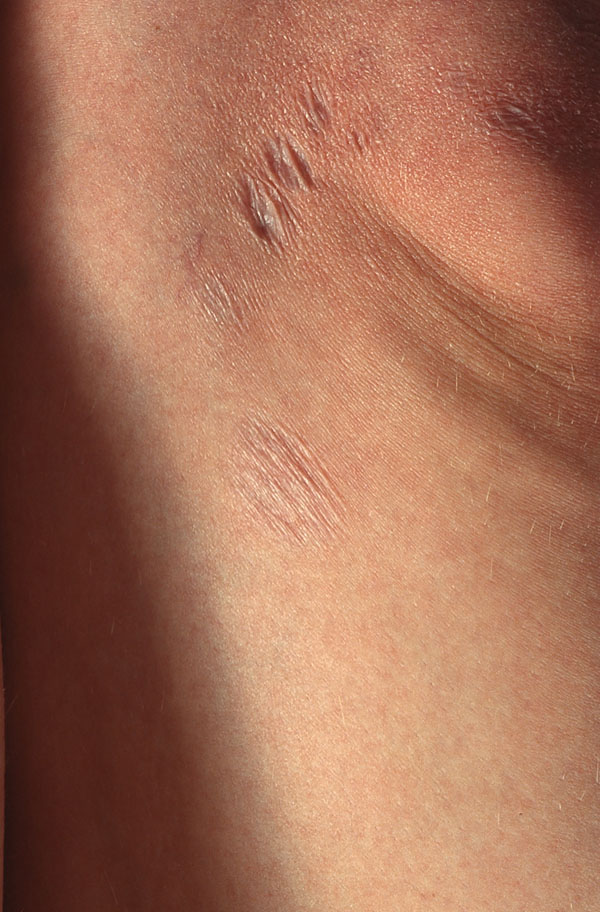
Anetoderma at the border of an ACA lesion after antibiotic therapy.
Anetoderma was also observed with and without association to ACA proven by ELISA, IB and amplification of B. afzelii-specific DNA from skin [89]. Similarly, Bauer et al. reported anetoderma with positive PCR using the 23 S ribosomal RNA gene and increased IgG antibodies against B. burgdorferi by IB [90].
Congenital anetoderma was reported by Aberer and Weißenbacher in a newborn showing atrophic livid patches on the chest with progression in the first weeks of life. Antibodies against B. burgdorferi were negative in the baby and the mother, so LB was not proven. Treatment with ceftriaxone for 14 days stopped the progression and there was no wrinkling. Anetoderma so was not proven; the lesions rather correspond to atrophic morphea since deep structures like fat and muscle were also involved [91].
The pathogenic relationship between ACA and anetoderma is striking since the histological changes are very similar and both diseases have been described in association [89]. Atrophic wrinkled patches can remain after treatment of ACA (Fig. 6). Atrophic elastic tissue is observed among the inflammatory infiltrate in ACA, and elastic fibers, having been engulfed in HLA-DR positive cells, are HLA-DR positive [24].
Anetoderma in Lyme borreliosis.
| Reference | Diagnosis | Detection method | Outcome |
|---|---|---|---|
| Aberer E [91] 1997 Austria |
Congenital anetoderma induced by intrauterine infection? | B. burgdorferi- antibodies neg. in mother and child | Complete resolution with ceftriaxone for 14 days with persisting atrophy of skin and fatty tissue, no recurrence after 8 years |
| Bauer J [90] 2003 Germany |
Anetoderma: another facet of Lyme disease | Positive PCR with 23S ribosomal RNA gene, positive B. burgdorferi IgG-antibodies | Doxycycline 100mg bid 30 days; no new lesions |
| Hofer T [89] 2003 Switzerland |
Anetoderma and LB: is there a pathogenetic relationship? (2 patients) |
B. afzelii-specific DNA, positive B. burgdorferi antibodies | Improvement after doxycycline 200mg 28-30 days in both patients |
| Trevisan G [112] 2008 Italy |
Anetoderma associated with Lyme disease | Nested PCR from skin biopsy and tissue culture positive; genotyping revealed B. afzelii | Penicillin G 20 Mill U/d in 4 doses 14 days; Amoxicillin bid 21 days; No further anetoderma lesions over a period of 6 months |
| Strunk T [88] 2011 Germany |
Anetoderma with positive Borrelia serology | Positive B. burgdorferi - IgM antibodies | Doxycycline 100mg bid; progression arrested |
CLINICAL REPORTS OF THE ASSOCIATION OF SCLEROTIC LESIONS WITH B. BURGDORFERI INFECTION AND THE EFFECT OF ANTIBIOTIC TREATMENT (Table 3)
After penicillin treatment, a seropositive patient with morphea profunda on the flexor aspects of the thigh was remarkably improved [9]. Borrelia infection was proven by detection of specific B. burgdorferi DNA and IgG antibodies in a patient with sclerodermiform skin lesions of the extremities and polyneuropathy with improvement of dermatologic and neurologic symptoms after ceftriaxone [65].
Repeated oral treatment with penicillin V and/or doxycycline for 20-30 days was effective in 6 among 30 patients with different types of morphea [25]. The lilac ring disappeared and the sclerotic plaques gradually smoothened in 8 patients treated with penicillin G 10 Mill IU intravenously bid for 14 days. Three patients unresponsive to systemic penicillin therapy responded to ceftriaxone. Two patients treated with ceftriaxone 2g for 15 days and 1 patient treated with intravenous penicillin recovered completely. All 6 seronegative patients with atrophodermia Pasini-Pierini and 1 seropositive patient with livid atrophic patches showed progression in spite of antibiotic therapy.
Wackernagel et al. reported a patient with positive ANA and CENP-B antibodies and abrupt onset of diffuse erythemas and doughy swellings involving the face and the upper trunk, followed by thickening and induration of the skin mimicking diffuse systemic scleroderma. B. burgdorferi infection was proven by repeatedly positive urine PCR and positive B. burgdorferi ELISA confirmed by IB. The patient responded rapidly to intravenous treatment with ceftriaxone 2g for 20 days [92].
Gubertini et al. observed an association of LSA, scleroderma en coup de sabre and Lyme borreliosis shown by positive PCR in the skin biopsy, blood and urine. Improvement was achieved with two courses of ceftriaxone 2g for 21 days in combination with UVA-1/vitamin E therapy [93]. Fifteen lichen sclerosus patients from the United States without evidence of a Borrelia infection were treated for 3-21 months with either intramuscular benzathin penicillin, cephalosporins, or oral antibiotics in an observational study [94]. All patients showed a significant response within a few weeks. Treatment of lichen sclerosus with either intramuscular ceftriaxone every 3 weeks or intramuscular penicillin every 2-3 weeks is recommended.
Prinz et al. observed a correlation of Borrelia-associated morphea diagnosed by serology with positive ANA [40]. Twenty of 90 patients were seropositive although some patients had only IgM antibodies, which can be interpreted as non-specific. Antibiotic treatment with penicillin G or ceftriaxone for 21 days improved sclerosis and arrested progression. As all patients had concomitant UV therapy, the effect of antibiotic treatment cannot be assessed objectively.
Treatment response in morphea.
| Reference | Clinical cases | Detection method | Outcome |
|---|---|---|---|
| Büchner SA [9] 1990 Switzerland |
Morphea profunda | Positive antibodies | Penicillin; remarkable improvement |
| Wackernagel A [92] 2005 Austria |
Acute exacerbation of SSc in Bb infection | Positive PCR in urine; positive ELISA and immunoblot;genotyping B. afzelii | Complete resolution after antibiotic treatment with ceftriaxone 2g for 21 days |
| Kaya G [113] 2001 Switzerland |
Chronic borreliosis presenting with morphea- and LSA-like cutaneous lesions | Positive PCR (290 bp-specific primer); positive IgM and IgG antibodies by immunoblot | Ceftriaxone 2g for 21 days; improvement after 7 days |
| Prinz JC [40] 2009 Germany |
Multiple plaques and linear morphea | Positive IgM antibodies, Positive ANA | Ceftriaxone 2g for 21 days + UVA 1; no further progression, no relapse, reduced sclerosis |
| Prinz JC [40] 2009 Germany | Generalized morphea | Positive IgG antibodies, positive ANA, U1RNP-, Sm-antibodies |
Penicillin G 10 mega units bid for 21 days + PUVA bath; reduction of sclerosis, relapse after 16 months |
| Prinz JC [40] 2009 Germany |
Morphea with multiple plaques | Positive IgM antibodies, Positive ANA | Oral amoxicillin 500mg tid for 14 days + UVA 1; relapses |
| Gubertini N [93] 2011 Italy |
LSA, scleroderma en coup de sabre | Positive PCR in blood, tissue, urine | 2 cycles ceftriaxone 2g 21 days + UVA 1 and Vit. E; improvement |
| Miglino B [95] 2012 Italy |
Localized scleroderma unius lateri and B. burgdorferi infection | Positive IgM antibodies, negative IgG antibodies Negative PCR | 2 courses of benzathin penicillin 12 Mill IU/ day for 20 days; clear improvement after 4 months, softening of deep tissues |
The response to antibiotic treatment in morphea and LSA is not dependent on a confirmed Borrelia infection. Borrelia DNA from peripheral blood and skin tissue was negative and only B. burgdorferi IgM antibodies were positive in a patient with left-sided painful scleratrophic plaques, progressive weakness and limb hypotrophy. After two courses of benzathin penicillin, there was clear improvement of skin lesions and softening of deep sclerotic plaques [95].
DISCUSSION
Buechner et al. suggest that ACA is a chronic T-cell mediated disease leading to severe tissue damage with atrophy of the epidermis and connective tissue [96]. Fibrosis is a typical reaction pattern seen in LB, not only in the skin but also in other tissues like the heart [22, 97]. Heart ventricles of adult Macaca mulatta monkeys show large deposits of collagen and the presence of Borrelia after inoculation with the B. burgdorferi sensu stricto N40 strain [97].
B. burgdorferi has high affinity to collagen and could be detected among collagen fibers in ACA by electron microscopy [23]. Borrelia and their degenerative products were frequently located along or between collagen bundles in morphea, partially or completely hidden if not visualized in the correct section plane as shown by FFM [59] or forming colonies in the collagen in erythema migrans, as observed by videomicroscopy [57].
This affinity of Borrelia to collagen was also shown in in vitro experiments with invasion and colonization of native type I collagen lattices [98, 99]. Binding of B. burgdorferi to adhesins such as the glucosamine-binding protein [100], fibronectin-binding protein [101, 102] and the proteoglycan decorin has been described [103]. Furthermore, B. burgdorferi possesses collagenolytic activity and plasmin-coated Borreliae degrade the extracellular matrix [104, 105]. It was suggested by Ackerman that fibrosis results mainly as a consequence of collagen destruction. Fibroblasts attempt to repair the defect but they never completely restore the original state [106]. Similarly, an increase in elastic fibers in LSA may reflect a process to repair the degraded upper dermal elastic fibers [26]. Elastic fibers are also markedly reduced in the upper and very prominent in the deeper dermis in ACA [24]. Damage of elastic and collagen fibers by B. burgdorferi has been described [107].
Laboratory methods to detect LB have to be interpreted cautiously as some test results could be misinterpreted. ELISA and IB employ different antigens and techniques that vary in their sensitivity and specificity [16], and two-tiered testing is not routine. PCR is performed with different primers to detect DNA or RNA and various sequencing methods, so that the results of these tests are hardly comparable; interpretation methods also differ. In some studies, patients were seropositive by anti - B. burgdoferi IgM antibodies only; these are not specific for a Borrelia infection when IgG seroconversion does not follow [40, 95, 108], or when the ELISA cannot be confirmed by IB [55]. The association between morphea and a Borrelia infection was reported from countries that tend to have a higher frequency of B. burgdorferi seroprevalence in the general population [16]. The results of laboratory findings and their interpretation are contradictory. When there was a small number of seropositive morphea patients, the serologic data were interpreted as a possible cross reaction, as in a paper by Hoesly et al., who reported 6/32 positive patients from the USA [109]. No Borrelia-associated morphea cases were reported in the United States. On the other hand, Granter et al. in Boston detected Borrelia in tissue sections by silver staining or by a polyclonal antibody in 4 patients with diffuse fasciitis [80].
It takes experience to identify B. burgdorferi on histological slides from among the different Borrelia forms, since the morphology of Borrelia in tissues is very heterogeneous [57]. Borrelia can only be visualized with special techniques such as videomicroscopy or focus floating microscopy, and time resources also limit this special diagnostic technique. The number of Borreliae can be very low, so that a negative finding does not rule out a Borrelia infection, and the antibodies used have different sensitivities. Borrelia can also be identified in seronegative morphea and LSA cases. Moreover, B. burgdorferi was detected immunohistochemically, not only in skin biopsies of morphea and LSA but also in several other diseases like cutaneous B-cell lymphoma, lymphocytic infiltration, granuloma anulare, interstitial granulomatous dermatitis, as well as in isolated cases of cutaneous sarcoidosis, necrobiosis lipoidica and necrobiotic xanthogranuloma [110]. The specificity of these findings remains to be determined. Borrelia can survive in different tissues and visualizing this bacterium does not always mean that it is the pathogen causing the disease.
There are significant geographic differences in the reported scleratrophic Borrelia infections, with a higher prevalence in areas of middle Europe (Fig. 7) [6]. The results of most PCR-based studies do not argue for a significant association of B. burgdorferi with morphea and LSA, and PCR was only applied in limited studies. The contradictory findings might have discouraged researchers from any further search for an infectious origin of scleratrophic skin diseases. Treatment success with antibiotics has often been reported, but controlled studies have not so far been undertaken to prove their efficacy. Pencillin G has been successfully used for therapy of dermal fibrosis. The effect of penicillin has also been attributed to a non-antibiotic action involving collagen metabolism. Yet, the mechanism of the antifibrotic action of penicillin G is still unknown [114].
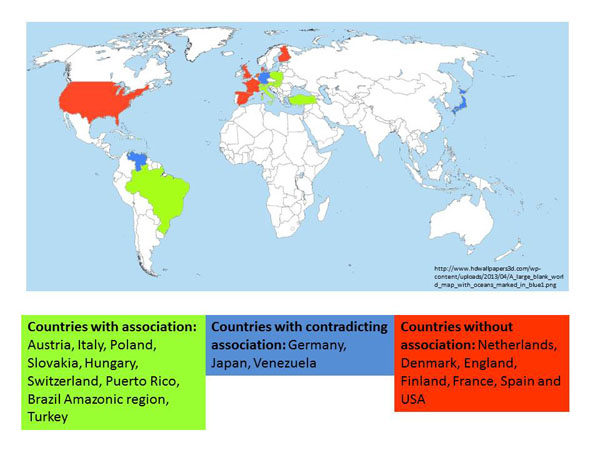
World-wide reported B. burgdorferi - associated scleratrophic changes.
CONCLUSION
The sclerotic/atrophic skin changes observed in LB vary, so several differential diagnoses might be relevant and should be distinguished from the putative LB-associated diseases (Table 4).
The possible relationship between scleroatrophic skin lesions with Lyme borreliosis is supported by 1) the similar clinical appearance of morphea with EM or ACA, 2) coexistence of morphea with ACA and/or LSA in the same patient, 3) the presence of a higher percentage of antibodies than in healthy controls, 4) positive PCR, 5) identification of Borrelia on histological sections, 6) culture of B. burgdorferi from skin lesions, and 7) the response to treatment of some patients. Treatment response was also reported from morphea cases without antibodies or without another proven borrelial origin. Since morphea is a heterogenous disease, another microorganism might be causing it. It is also possible that a Borrelia infection cannot be proven because the laboratory techniques are not sensitive enough. Anetoderma can also be associated with ACA. In this rare disease, single case reports have shown anti - B. burgdorferi antibodies, positive B. burgdorferi DNA and response to antibiotic treatment.
Differential diagnoses of scleratrophic skin diseases.
| Morphea - pseudoscleroderma | Atrophic skin diseases |
|---|---|
| Granuloma anulare | Granulomatous slack skin |
| Systemic nephrogenic fibrosis | Steroid atrophy |
| Radiation fibrosis | Neurogenic atrophy |
| Chronic graft-versus host disease | Progeria |
| Lipodermatosclerosis | Striae cutis distensae |
| Skleromyxoedema | Atrophic scarring |
| Skleredema diabeticorum | Atrophy in skin aging |
| Skleredema adultorum Buschke | Atrophodermia vermiculata |
| Eosinophilia-myalgia sndrome | |
| Toxic oil syndrome | |
| Porphyria cutanea tarda | |
| Progeria syndromes |
There is a strong relationship between specific geographic areas with reported Borrelia-associated morphea cases and with the predominant genotype. B. afzelii was the predominant species that was isolated from ACA and B. afzelii DNA has been detected predominantly in skin lesions. Another cause for the different results in hitherto published studies is that sclerotic lesions can be misinterpreted since the pseudoscleroderma of ACA can easily be mistaken for morphea.
The pathologic changes in scleratrophic skin lesions point to connective tissues damage, which can be caused by B. burgdorferi. A high affinity of Borrelia to collagen and degradation of fibers is the central process that has not yet received attention. It can be assumed that the scleratrophic skin lesions can be caused by Borrelia infection in certain countries where B. afzelii is endemic. The response to antibiotic treatment in cases where there was no proven association to a borrelia infection suggests that other microorganisms might be involved in scleratrophic dermatoses and suggests that these diseases must be of heterogeneous origin.
To prove the Koch`s postulates, transmission experiments that have been performed in EM, ACA and lymphadenosis cutis are missing for scleratrophic skin lesions. In future controlled multicenter studies with standardized laboratory techniques including placebo-controlled treatment studies should be performed in a well-defined morphea population to determine the role of B. burgdorferi in scleratrophic skin diseases.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
Declared none.


