RESEARCH ARTICLE
Assessment of the Degree of Skin Hypopigmentation in Patients with Vitiligo by using Mexametry
N.V. Deeva*, Yu.M. Krinitsyna, S.V. Mustafina, O.D. Rymar, I.G. Sergeeva
Article Information
Identifiers and Pagination:
Year: 2017Volume: 11
First Page: 53
Last Page: 58
Publisher ID: TODJ-11-53
DOI: 10.2174/1874372201711010053
Article History:
Received Date: 17/07/2017Revision Received Date: 04/09/2017
Acceptance Date: 17/09/2017
Electronic publication date: 13/10/2017
Collection year: 2017

open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: (https://creativecommons.org/licenses/by/4.0/legalcode). This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
This study is an assessment of the degree of skin hypopigmentation in patients with vitiligo.
Material and Methods:
The study followed 47 patients with vitiligo (33 female and 14 male patients; the average age was 38.0 ± 18.0 years). The mean disease duration was 15.5 ± 14.1 years. We determined the melanin levels in the patches of vitiligo and on the healthy skin of the face, trunk, and extremities by using mexametry.
Results:
High melanin levels were found in patches on and around the mouth. Melanin levels did not differ in vitiligo patches and on healthy skin of chins and buttocks. All patients had no melanin in the patches on their cheeks. Having vitiligo for a long time reduces melanin levels in the skin of the forehead. High melanin levels in healthy skin are associated with stored melanin in vitiligo patches in axillary areas, on the back, brachiums, and femurs.
Conclusions:
There are significant differences in the melanin levels in the vitiligo patches and healthy skin, which have specific features depending on the localization. Analysis of melanin levels may be useful in choosing a method and evaluating the effectiveness of the planned therapy.
1. INTRODUCTION
Vitiligo is a disease characterised by patches of skin hypopigmentation tending to peripheral growth and develops because of the disappearance of melanocytes or disruption of their functional activity [1].
Because the pathogenesis of vitiligo is still not fully understood, the methods of treating this disease are often ineffective, despite the application of evidence-based medicine. Therefore, in addition to conventional methods of diagnosis, instrumental analyses are important for studies of human skin in vivo, because these analytical tools are standardised and objectively reflect the distribution of pigment in the lesions. One such analytical method is mexametry. It allows researchers to quantify the distribution of the pigment in the skin of patients with vitiligo, to determine the characteristics of the disease in patients, and to monitor changes in the pigment content in the patches during the process of therapy.
2. MATERIAL AND METHODS
We studied 47 patients with vitiligo, 33 (70.2%) women and 14 (29.8%) men aged from 8 to 77 years with a mean age of 38.0 ± 18.0 years. The mean duration of the disease was 15.5 ± 14.1 years. This study was approved by Local ethics committee of Research Institute of Internal and Preventive Medicine – Branch of the Institute of Cytology and Genetics. The subjects in this study were recruited after obtaining written informed consent.
Twelve (25.5%) vitiligo patients had diffused telogen hair loss and 12 (25.5%) had onychodystrophy (which encompasses a wide spectrum of nail disorders). Thirteen (27.7%) patients had autoimmune thyroiditis, 4 (8.5%) had halo naevus, and 1 (2.1%) had psoriasis. Family history of the patients with the disease revealed that 14 (29.8%) had relatives with vitiligo, 3 (6.4%) had a family history of rheumatoid arthritis, and 1 (2.1%) had a family history of psoriasis.
Vitiligo was evaluated according to the Vitiligo Global Issues Consensus Conference (2012) Vitiligo Disease Activity score (VIDA) disease activity classification scale [2-4]. The generalised form of vitiligo was observed in 26 patients (55.3%), mixed in 9 (19.1), acrofacial in 4 (8.5%), focal and universal in 3 (6.4%) each, and segmental in 2 (4.3%). The progressing type of vitiligo was noted in 39 patients (83.0%), while stable and unstable forms were noted in 4 (8.5%) patients each.
We determined melanin levels by mexametry. The measurement of melanin distribution in the skin was based on the definition of light absorption. The range of values was denoted by 1-100 conventional units (c.u.). The error of this method does not exceed 0.1%.
We determined melanin levels in the patches of vitiligo and healthy skin at 19 locations: forehead, eyelids, cheeks, lips, chin, neck, breast, axillary areas, back, buttocks, inguinal folds, brachiums, elbows, forearms, the back of brushes, femurs, knees, shins, and the back of the feet. A total of 346 measurements were performed in the patches of vitiligo and 474 measurements on healthy skin.
We defined skin type using the Fitzpatrick skin type scale classification. The majority of patients with vitiligo had phototype 2 in 24 (51.1%) patients and phototype 3 in 16 (34.0%) patients; phototype 4 occurred in 3 (6.4%) and phototypes 1 and 5 occurred in 2 (4.3%) patients.
Statistical data processing was performed using the program Statistica 10.0 (StatSoft, Tulsa, OK, USA). The results are presented as the mean value of the indicator and its standard deviation (M ± SD) or percentage incidence. To assess group differences, Student's t-test was calculated and Spearman's rank correlation coefficient (r) was used for the characterisation of the binding forces between the parameters. Test results that produced P values < 0.05 were regarded as statistically significant.
3. RESULTS
The patches of vitiligo were diagnosed on the back of brushes in 32 (68.1%) patients; on the eyelids in 29 (61.7%); on the axillary areas in 27 (57.5%); on the breast and on the back of the feet in 25 (53.2%); on the forearms, shins, and back in 23 (49.0%); on the knees and elbows in 20 (42.6%); on the inguinal folds in 18 (38.3%); on the femurs in 15 (31.9%); on the neck, chin, and lips in 12 (25.5%); and on the brachiums in 10 (21.3%). Hypopigmented patches were seen less often on the buttocks in 9 (19.2%), on the cheeks in 6 (12.8%), and on the forehead in 5 (10.6%).
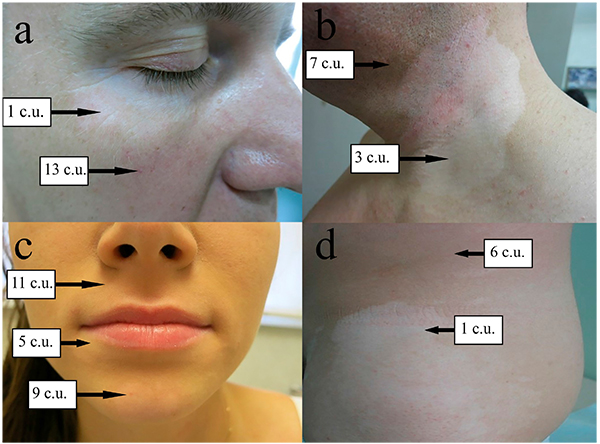
The melanin levels in healthy skin of patients with vitiligo were approximately 6-13 c.u. on the eyelids, cheeks, lips, chin, neck, back, buttocks, axillary areas, and inguinal folds, but the melanin levels in the vitiligo patches in these areas differed significantly Fig. (1). The melanin levels in the vitiligo patches and healthy skin did not differ significantly on the chin and buttocks (P > 0.05) Table (1). Melanin was almost absent in vitiligo patches on the cheeks (1 c.u.) in all patients.
| Localisation | Melanin levels, c.u. | |
|---|---|---|
| healthy skin | vitiligo patches | |
| Forehead | 15.8 ± 4.5 | 2.4 ± 2.2*** |
| Eyelids | 9.2 ± 8.0 | 1.1 ± 0.6*** |
| Cheeks | 11.3 ± 5.6 | 1.0 ± 0.0*** |
| Lips | 12.1 ± 4.6 | 8.1 ± 5.0* |
| Neck | 12.5 ± 7.7 | 1.3 ± 0.6*** |
| Breast | 5.0 ± 5.4 | 1.5 ± 2.2** |
| Axillary areas | 13.1 ± 9.0 | 2.8 ± 4.1*** |
| Back | 12.7 ± 11.1 | 2.4 ± 2.7*** |
| Inguinal folds | 13.8 ± 7.8 | 3.7 ± 4.8*** |
| Brachiums | 16.5 ± 12.3 | 2.0 ± 1.8** |
| Elbows | 17.7 ± 11.2 | 2.2 ± 2.3*** |
| Forearms | 19.0 ± 10.9 | 3.0 ± 5.1*** |
| The back of brushes | 16.7 ± 11.5 | 1.5 ± 1.3*** |
| Femurs | 14.1 ± 10.7 | 4.8 ± 3.6** |
| Knees | 19.2 ± 7.6 | 5.5 ± 5.6*** |
| Shins | 16.4 ± 10.4 | 3.3 ± 3.1*** |
| The back of feet | 21.7 ± 9.8 | 3.2 ± 4.0*** |
| Chin | 13.7 ± 6.0 | 10.8 ± 6.9 |
| Buttocks | 6.6 ± 7.4 | 2.7 ± 2.8 |
Table (2) shows the correlation between melanin levels in vitiligo patches and the duration of the disease. We found a negative correlation (r = -0.89; P < 0.05) between the melanin levels in vitiligo patches of the forehead and the disease duration. Thus, a longer duration of disease affects the decrease in the melanin level in the skin of only the forehead. We also found a positive correlation, but not a significant one, between the duration of vitiligo and the melanin levels in the patches on the chin, neck, buttocks, femurs, axillary areas, and inguinal folds. This implies that a high melanin level is preserved better in these areas compared to that at other locations over the course of time.
| Localisation | The correlation coefficient (r) with a duration of disease |
|---|---|
| Forehead | -0.89* |
| Eyelids | -0.14 |
| Lips | -0.07 |
| Chin | 0.14 |
| Neck | 0.15 |
| Breast | -0.08 |
| Axillary areas | 0.08 |
| Back | -0.33 |
| Buttocks | 0.05 |
| Inguinal folds | 0.02 |
| Brachiums | -0.32 |
| Elbows | -0.35 |
| Forearms | -0.06 |
| The back of brushes | -0.13 |
| Femurs | 0.12 |
| Knees | -0.21 |
| Shins | -0.16 |
| The back of feet | -0.02 |
Table (3) show the correlation between melanin levels in vitiligo patches and healthy skin in the corresponding locations. We found a positive correlation between vitiligo patches and the following body locations: the axillary areas (r = 0.55; P < 0.05), on the back (r = 0.61; P < 0.05), on the brachiums (r = 0.68; P < 0.05), and on the femurs (r = 0.63; P < 0.05).
| Localisation in vitiligo patches and healthy skin | The correlation coefficient (r) |
|---|---|
| Forehead | 0.87 |
| Eyelids | 0.04 |
| Lips | 0.27 |
| Chin | 0.29 |
| Neck | -0.21 |
| Breast | 0.37 |
| Axillary areas | 0.55* |
| Back | 0.61* |
| Buttocks | 0.74 |
| Inguinal folds | 0.28 |
| Brachiums | 0.68* |
| Elbows | 0.09 |
| Forearms | -0.04 |
| The back of brushes | 0.09 |
| Femurs | 0.63* |
| Knees | 0.44 |
| Shins | -0.04 |
| The back of feet | -0.28 |
Thus, we found that high melanin levels relative to healthy skin is associated with a high preservation of melanin in the hypopigmented patches in the axillary areas and on the back, brachiums, and femurs.
4. DISCUSSION
Vitiligo is a common skin disorder of depigmentation that can occur in any location of the body. The localisation of vitiligo on the trunk is an independent risk factor for patients with nonsegmental vitiligo developing thyroid dysimmunity, although very few researchers have studied this association using multivariate logistic regression [5]. In our study, 13 (27.7%) patients had autoimmune thyroiditis, 4 (8.5%) had halo naevus, and 1 (2.1%) had psoriasis. In our study, the progressing type of vitiligo was noted in 39 patients (83.0%), while stable and unstable forms were noted in 4 (8.5%) patients each. Among our patients, most of them had a nonsegmental vitiligo with varying degrees of prevalence and a progressive process of the disease, probably explaining one of the reasons for patients' admission to the method with this disease.
There are conflicting and insufficient data on the location in the vitiligo patches. Thus, in a study of 21 patients whose mean age was 44.3 years, the assessment of vitiligo patches was carried out only in 7 locations: on the trunk (28.6%), legs (19.1%), feet (14.3%), hands (14.3%), the back of brushes (9.5%), wrists (9.5%) and face (4.8%) [6]. We determined melanin levels in the patches of vitiligo and healthy skin at 19 locations. The patches of vitiligo were most often diagnosed on the back of brushes, on the eyelids, on the axillary areas, on the breast and on the back of the feet in our study.
The presence of periorbital vitiligo was significantly related to the ocular findings, revealing concordances between periorbital and genitalial localisations of vitiligo [7].
We found that the highest average melanin levels in vitiligo patches on the chin were 10.8 ± 6.9 c.u. and around the mouth (lips) were 8.1 ± 5.0 c.u. The melanin levels in vitiligo patches and healthy skin were not significantly different (P > 0.05) on the chin and buttocks. The melanin levels in vitiligo patches on the cheeks were absent in all patients.
We found that a longer duration of disease affects the decrease in the melanin level in the skin of only the forehead (r = -0.89; P <0.05). We also found that high melanin levels in healthy skin are associated with a high preservation of melanin in the hypopigmented patches in the axillary regions (r = 0.55; P <0.05) and on the back (r = 0.61; P <0.05), brachiums (r = 0.68; P <0.05), and femurs (r = 0.63; P <0.05).
The evaluation of the effectiveness of the planned therapy is important for determining the predictions. However, there is currently no standardized method for measuring vitiligo damage. Objective and non-invasive methods for measuring and controlling the degree of pigmentation compared to the surrounding normal skin can be useful for assessing the effectiveness and monitoring of treatment [7]. Reflectance spectrophotometers have been used for many years to measure the level of skin pigmentation. Diffuse reflection spectroscopy (DRS) can be a suitable method for measuring skin color and melanin content of human skin in out [8].
A rather large number of studies on instrumental methods for studying human skin in vivo have been carried out, which are more standardized and therefore more objective. The method of mexametry was used to quantify the allergic or inflammatory reaction caused by UV damage, as well as to confirm the diagnosis of hemangioma, also used to determine the degree of skin tanning, phototype, evaluation of the effectiveness of bleaching procedures, to confirm the diagnosis of melanoma, individual studies to assess the effectiveness of treatment vitiligo after the procedures. However, data on the amount of melanin in all locations in vitiligo patches and on healthy skin, сorrelation between melanin levels in vitiligo patches and duration of disease, сorrelation between the melanin levels in vitiligo patches and healthy skin in the corresponding localisation, together with the clinical features of the disease, are obtained for the first time.
We found that the visual assessment of depigmentation in some cases does not correspond to the melanin levels in the patches of vitiligo. With an equal indirect visual assessment of depigmentation in the patches of vitiligo, melanin levels may be equal to 1 с.u. (absence of melanin), 3 c.u. and even 10 c.u. This requires further study regarding the choice of therapy in these areas of the skin.
CONCLUSION
There are significant differences in the melanin levels in the vitiligo patches and healthy skin, which have specific features depending on the localization. Analysis of melanin levels may be useful in choosing a method and evaluating the effectiveness of the planned therapy.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No Animals/Humans were used for studies that are base of this research.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.