All published articles of this journal are available on ScienceDirect.
Prospective Pilot Evaluation of the Efficacy and Safety of Topical Ingenol Mebutate Gel for Localized Patch/Plaque Stage Mycosis Fungoides
Abstract
Background:
Mycosis Fungoides (MF) is the most frequent type of the primary cutaneous NK/T-cell lymphomas. Ingenol mebutate (IM) displays in vitro pro-apoptotic properties on neoplastic lymphocytes.
Objectives:
To evaluate the efficacy and safety of IM gel as topical treatment for MF.
Materials and Methods:
Ten male patients with longstanding classic type MF (n=9) and follicular MF (FMF; n=1), T2bN0M0B0, stage Ib, resistant to systemic methotrexate or acitretin therapies for at least 3 months, were included in this pilot study. In these patients, 11 target patch/plaque stage lesions with an area ≤ 25 cm2 were selected for IM therapy (0,05%, 2 weekly applications). The primary endpoint was the improvement of the CAILS scores. Biopsies were performed before and after treatment from 10 target lesions. Relapse rates were evaluated at 6 months.
Results:
The mean CAILS score of treated target lesions was reduced by 58.2%. The mean erythema, scaling and plaque elevation scores were improved by 73.6%, 93.9% and 97.9% (p<0.0001), respectively, while the lesion size remained unchanged (p=0.34). A complete or partial clearance of histological and immunohistochemical features was observed in 6/10 (60%) and 4/10 (40%) of the MF or FMF target lesions, respectively. Monoclonal TCR rearrangement was evidenced in 100% (7/7) of the patients and in 3/7 (43%) after treatment. The relapse rate at 6 months was 18%. All the patients experienced burning sensations, oozing and crusting.
Conclusion:
IM gel warrants further investigation and development as a potential topical treatment for localized patch/plaque stage MF and FMF.
1. INTRODUCTION
Ingenol Mebutate (IM) is a macrocyclic diterpene ester and originates from the plant Euphorbia peplus (PEP005, 0,015%, 0,05%, Picato° gel, Leo Pharma) [1, 2]. For decades the sap from E. peplus has been used as traditional medicine for various types of skin cancer [1, 2]. Both the EMA and the FDA recognize IM gel as a field-directed treatment for Actinic Keratosis (AK) [1, 2]. IM also revealed to be an efficacious topical treatment for other cutaneous (Fig. 3) epithelial cancers, including superficial basal cell carcinoma [3-5], Bowen’s disease [6, 7] and murine cutaneous squamous cell carcinoma [8]. IM has also demonstrated activity against melanoma cell lines in vitro [9, 10] and, in vivo, for Dubreuilh’s melanoma of the face [11].
IM and other ingenol derivatives activate multiple signaling pathways in cancer cells, including the intracellular pro-apoptotic Protein Kinase C (PKC) δ, α and ϵ, NF-κB1, ERK, JNK and Akt pathways [10, 12]. The activation of these PKC’s leads to a rapid cancer cell apoptosis and a neutrophil-mediated immunostimulation of antibody-dependent cellular cytotoxic response [13]. The IM-induced pro-inflammatory and pro-apoptotic storm could potentially be of therapeutic benefit for atypical lymphocytes. Ingenol 3-angelate has previously been demonstrated to be effective against murine B-lymphoma cells [12]. IM induced apoptosis in acute myeloid leukemia cells by activating the PKC isoform PKCδ [14].
The classic type of Mycosis Fungoides (MF) is the most frequent form of the primary Cutaneous T/NK Cell Lymphomas (pCTNKCL). MF follows typically an indolent cutaneous disease course and is an ideal candidate for skin-directed therapies. Its folliculotropic variant (FMF) is a more rare and late-stage FMF may present a more aggressive disease course [15-18].
In the view of the action mechanisms of IM, we were interested to evaluate whether IM gel 0,05% had a potential effect on patch/plaque stage lesions in MF and FMF patients that were previously resistant to systemic methotrexate or acitretin.
This prospective pilot study assessed the improvement of the CAILS (Composite Assessment of Index Lesion Severity) score [19] and the histological cure rate 2 months after using IM gel on 11 target lesions in 10 patients with histologically confirmed, longstanding patch/plaque stage MF and FMF.
2. MATERIALS AND METHODS:
2.1. Institutional Ethics
This prospective pilot study was performed in accordance with the ethical standard of the University Hospital Committee on institutional human experimentation and with the Helsinki Declaration of 1975, and amended in 2013. The aims of the study and all the procedures were explained to all the patients and all signed an informed consent.
2.2. Patient Selection
At baseline, the patients had to present patch/plaque stage lesions that were not responding to at least 3 months of oral methotrexate or acitretin treatments. Patients were selected between Feb and May 2017. The individual patient demographics, including age, gender, type of underlying MF, TNMB staging and disease stage, mSWAT (modified Severity-Weighted Assessment Tool) score of the underlying MF at baseline, as well as the site of the selected target lesion are summarized in Table 1. All the patients had longstanding patch/plaque type classic type MF (n=9) or FMF (n=1). The diagnosis of classic type MF or FMF was made histologically at least 4 years before inclusion in the study and sustained by an immunohistochemical CD3+, CD4+, CD45R0+ and CD8- expression profile of the MF infiltrate and by the presence of a monoclonal T-Cell Receptor (TCR) rearrangement (10/10 cases).
| Patient | Gender, Age | Type, TNMB, Stage, mSWAT | Target Lesion Site(s) | Duration of MF (years) |
|---|---|---|---|---|
| 1 | M, 64 years | FMF, T2bN0M0B0, Ib, 30 | Perineal | > 7 |
| 2 | M, 52 years | MF, classic type T2bN0M0B0, Ib, 82 | Left arm | > 5 |
| 3 | M, 70 years | MF, classic type T2bN0M0B0, Ib, 56 | Left arm | > 8 |
| 4 | M, 62 years | MF, classic type T2bN0M0B0, Ib, 84 | Right arm | > 5 |
| 5 | M, 73 years | MF, classic type T2bN0M0B0, Ib, 74 | Chest | > 4 |
| 6 | M, 76 years | MF, classic type T2bN0M0B0, Ib, 88 | Knee | > 5 |
| 7 | M, 79 years | MF, classic type T2bN0M0B0, Ib, 48 | Knee | > 8 |
| 8 | M, 80 years | MF, classic type T2bN0M0B0, Ib, 42 | Axillar | > 4 |
| 9 | M, 65 years | MF, classic type T2bN0M0B0, Ib, 78 | Buttock | > 5 |
| 10 | M, 72 years | MF, classic type T2bN0M0B0, Ib, 44 | Inguinal Abdominal |
> 6 |
| Mean age: 69 years |
Mean mSWAT score: 59.6 | Mean duration of MF: 5.7 years |
2.3. Target Site Selection
Per patient, one or two target patch/plaque type lesion(s) of 3 to 7 cm in diameter were selected. Target lesions had to be present without any clinical changes since at least 3 months. Target lesions exceeding 25 cm2 were excluded, as manufacturer instructions say to treat an area of not more than 25 cm2.
2.4. Control Site Selection
One or more control lesions in the immediate vicinity of the target lesion were selected. The size of the control lesions was up to 25 cm2. The control lesions had to present a clinical highly similar aspect as the target lesion.
2.5. Photographic Recording
Photographic recordings were made of every target lesion and control lesion(s) under standard conditions at baseline (T0) and at 2 months after therapy (T2).
2.6. Treatment Application
One tube of IM gel (0,05%, 235 µg of IM in 0,47 g of gel, Leo Pharma°) was applied to the target lesion at day 1 and day 7. The 0,05% formulation was selected, as none of the target lesions was located on the face. Patients were informed concerning potential adverse effects and how to manage them. The one-week interval between IM applications was selected to avoid too severe cutaneous adverse reactions as sometimes observed in AK patients with the 2 consecutive days IM application regimen, and furthermore, to be able to judge the severity of the cutaneous reactions after one single application and to reduce the severity the IM-treatment-related adverse effects. The control lesions did not receive any topical treatment. No other topical treatments were allowed for the target and control lesions during the entire study. The previous oral treatments were interrupted three months before inclusion.
2.7. Histology, Immunohistology and TCR
A 4-mm cutaneous punch biopsy of the target lesion was obtained under local anesthesia, fixed in neutralized formalin 4% and embedded in paraffin before and 2 months after treatment. Histopathological examination for MF criteria was performed. The histologic scoring system was the following: no change compared to baseline, a partial improvement (at least 75%) of the MF infiltrate or a total resolution (with or without persistence of post-inflammatory lymphohistiocytic infiltrate and/or post-inflammatory hyperpigmentation). Immunohistochemistry searched for CD3, CD4, CD45R0 and CD8 expressions. Immunohistological scoring was as follows: no change compared to baseline, partial resolution (at least 75% improvement) and total healing in terms of CD3, CD4, CD45R0 and CD8 expression. TCR monoclonal rearrangements were evaluated as present or absent at baseline and 2 months after treatment from 7 target lesions. No histology was performed for the control lesions.
2.8. CAILS Score
The primary endpoint in this pilot setting was the mean CAILS (Composite Assessment of Index Lesion Severity) score improvement, expressed in percentage (T0-T2), evaluating the erythema, scaling, plaque elevation and hyper/hypopigmentation scores (all: ranging from 0 (none) to 8 (very severe)) as well as the lesion size (1: up to 4 cm2 up to maximum 4: 16-25 cm2), as used for individual lesion evaluation, before (T0) and 2 months (T2) after treatment of the target and control lesions [19].
2.9. Local Adverse Effects
Cutaneous local adverse effects assessment was performed 5 days after the first and second IM application. The scoring of burning sensations, oozing, and crusting used the following scale: 0: none, 1: mild, 2: moderate and 3: severe. No evaluation of eventual systemic adverse effects was included in this pilot study as no such effects were previously reported in AK studies [1-5].
2.10. Recurrence Rate
The clinical relapse rate of the target lesion was assessed 6 months after the initiation of the IM treatment. Relapse was defined as the re-appearance of the patch/plaque infiltration and/or erythema and/or scaling.
2.11. Statistical Analysis
A comparison of the mean relative differences (T0 and T2) between target and control was made using the Student test for the individual CAILS parameters, erythema, scaling, plaque elevation, hyper/hypopigmentation and lesion size (L. Seidel).
3. RESULTS
3.1. Histology, Immunohistology and TCR
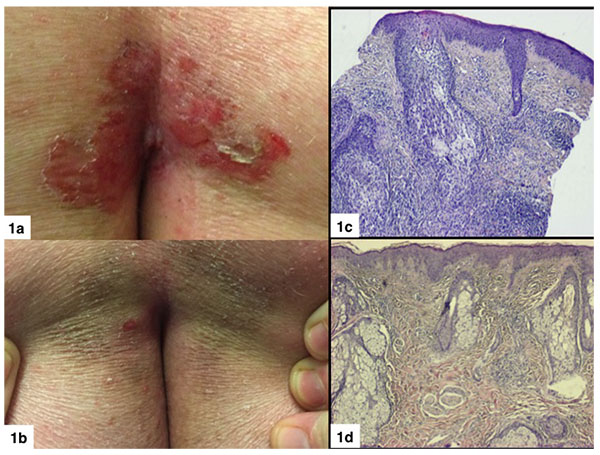
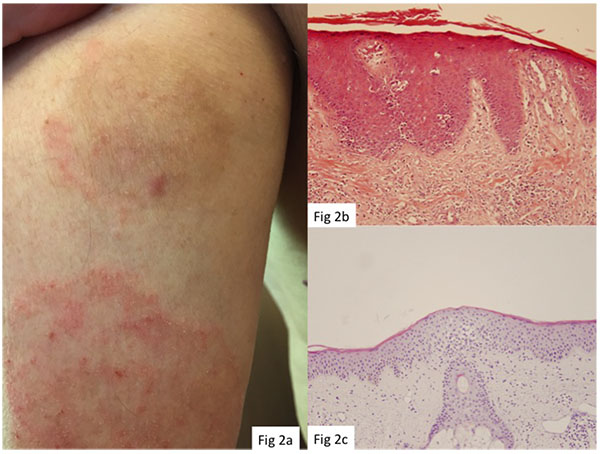
Punch biopsies were obtained from 10 target lesions before IM treatment (T0) and at 2 months after treatment (T2). Histopathological examination revealed a complete or partial absence of histological signs and immunohistochemical features of MF or FMF in 6/11 (55%) and 4/11 (36%) target lesions, respectively (Figs. 1 and 2). Monoclonal TCR rearrangement of the target lesions before treatment was positive in 7/7 (100%) cases and in 3/7 (43%) at 2 months post-treatment.
3.2. CAILS Scores
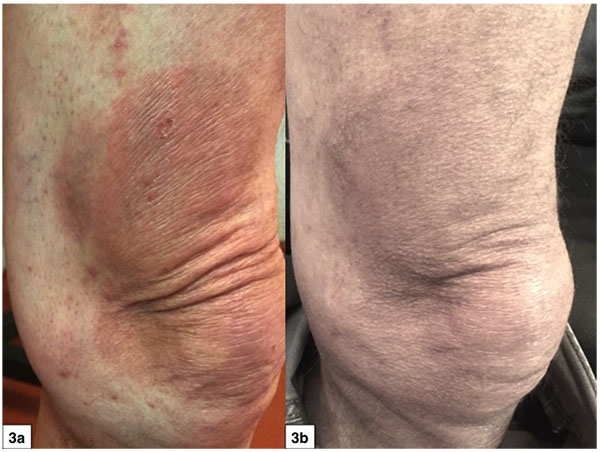
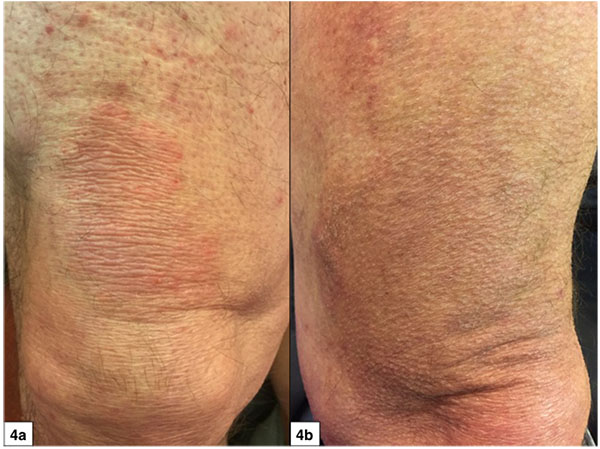
The study results are listed in Tables 2 and 3. The mean overall CAILS score (T0-T2) was reduced by 58.2%. The erythema, scaling and plaque elevation mean scores (T0-T2) were improved by 73.6%, 93.9% and 97.7% respectively, all statistically significant (p<0.0001). No hyper or hypopigmentation was observed at baseline. Mild to moderate hyperpigmentation appeared in 8/11 (73%) of the target lesions. No reduction in lesion size was observed in any of the target and control lesions (Figs. 1-4).
| Variable | N | Mean | SD | SE | Min | Q1 | Median | Q3 | Max | Student p-value |
|---|---|---|---|---|---|---|---|---|---|---|
| ErythdT0T2 | 11 | 3.818 | 1.401 | 0.42 | 2.0 | 3.0 | 4.00 | 5.0 | 6.0 | <.0001 |
| ScalingdT0T2 | 11 | 3.273 | 1.421 | 0.43 | 1.0 | 2.0 | 4.00 | 4.0 | 6.0 | <.0001 |
| PlaquedT0T2 | 11 | 3.909 | 1.221 | 0.37 | 2.0 | 3.0 | 4.00 | 4.0 | 6.0 | <.0001 |
| HyppigmdT0T2 | 11 | -1.636 | 1.433 | 0.43 | -4.0 | -2.0 | -2.00 | 0.0 | 0.0 | 0.0036 |
| SizedT0T2 | 11 | 0.000 | 0.000 | 0.00 | 0.0 | 0.0 | 0.00 | 0.0 | 0.0 | |
| TotaldT0T2 | 11 | 9.364 | 2.580 | 0.78 | 6.0 | 7.0 | 9.00 | 12.0 | 14.0 | <.0001 |
| Variable | N | Mean | SD | SE | Min | Q1 | Median | Q3 | Max | Student p-value |
|---|---|---|---|---|---|---|---|---|---|---|
| dErythpT0T2C | 11 | 73.636 | 18.148 | 5.47 | 50.0 | 50.0 | 75.00 | 83.3 | 100.0 | <.0001 |
| dScalingpT0T | 11 | 93.939 | 10.601 | 3.20 | 75.0 | 83.3 | 100.00 | 100.0 | 100.0 | <.0001 |
| dPlaquepT0T2 | 11 | 97.727 | 7.538 | 2.27 | 75.0 | 100.0 | 100.00 | 100.0 | 100.0 | <.0001 |
| dHyppigmpT0T | 0 | . | . | . | . | . | . | . | . | |
| dSizepT0T2C0 | 11 | 3.030 | 10.050 | 3.03 | 0.0 | 0.0 | 0.00 | 0.0 | 33.3 | 0.34 |
| dTotalpT0T2C | 11 | 58.235 | 12.910 | 3.89 | 37.5 | 46.2 | 60.00 | 68.3 | 75.0 | <.0001 |
3.3. Recurrence
After a follow-up of 6 months after treatment, 2 out of 11 target lesions (18%) presented de novo erythema, slight infiltration and scaling, suggestive of MF recurrence. The hyperpigmentation observed at the evaluation at 2 months was significantly attenuated in 10 out of 11 cases at 6 months.
No beneficial or detrimental effects were noted for the surrounding control lesions.
3.4. Adverse Effects
Five days after the first and second application of IM, all the patients experienced treatment-related adverse effects. The scores for burning sensations, oozing and crusting were 1.6, 0.7, 1, and 1.2, 0.3, 0.5, respectively. All the patients accepted well the IM-inherent adverse effects and were never a reason to interrupt the trial.
4. DISCUSSION
Although the precise action mechanisms of IM on neoplastic lymphocytes should be further investigated, the IM-induced pro-inflammatory and pro-apoptotic storm [12] through activation of the PKC isoform PKCδ is likely to be involved in the anti-neoplastic action on MF lymphocytes [14].
As the control lesions in the direct vicinity of the target lesions were unaltered one may suggest that IM only acts locally at the site of application and does not generate a more expanded immune effect. This is in contrast to topical agents such as resiquimod, inducing not only a loco-regional immune effect, but also a systemic immune stimulation by enhancing circulating dendritic cells [20]. On the other hand, other authors described an adjuvant effect of IM by showing that its action on subcutaneous tumors simultaneously generated anti-cancer CD8+ T-cells, able to regress metastases and distant metastases by upregulating CD80 and CD86 expression on dendritic cells and by stimulating CD8+ T-cell induction when co-delivered with a protein antigen [21].
This pilot study supports the hypothesis that the action spectrum of IM is not only limited to neoplastic keratinocytes and melanocytes, but also expands to neoplastic lymphocytes of MF.
Due to the relative difficulty of finding 3 highly similar test MF lesions (IM target lesion gel, placebo gel lesion and no treatment lesion) in one patient, we decided in this pilot setting not to include a placebo gel group. Previous studies with IM on AK demonstrated that IM was significantly superior to placebo gel in terms of partial and complete clearance and the median percentage reduction in baseline AK lesions for patients treated with IM gel ranged from 75% to 100% compared with 0% for vehicle gel (P < .0001 vs vehicle) [2]. Furthermore, the 13.3% placebo effect in the AK studies could also be attributed to the natural evolution of “coming and going” of AK’s. In addition, the vehicle gel could have had a keratolytic effect on the hyperkeratotic part of the AK’s, explaining a placebo effect, a phenomenon that does not exist in MF lesions.
Even if only 11 target lesions of 10 T2b MF patients were included in this pilot study, the results remain interesting, in particular in comparison to other available topical treatments. In fact, for T2 MF, the topical retinoids group, including bexarotene 1% gel, tazarotene 0,1% gel and alitretinoine 0,1% gel, presented a complete cure of 21%, 33% and 100% (one single patient), respectively [22-24]. The potent to very strong corticosteroids achieved a complete response in 25% in T2 MF patients [25, 26]. The topical immunomodulators such as imiquimod 5% cream presented a 50% cure rate [27] and resiquimod 0.03% and 0.06% gel presented a 30% cure rate in 12 IA-IIA MF patients [20]. Topical chemotherapies using mechlorethamine or carmustine in T2 MF patients achieved about a 50% complete response [28-31].
IM already achieved a good clinical response after 2 months versus 10-19 months with mechlorethamine or carmustine [28-32] or versus 20.1 months for bexaroten gel [22]. Hence, IM gel seems to act more rapidly compared to other topical agents.
That TCR monoclonality was still present in 3 of 7 target lesions at T2 is probably related to the high sensitivity of the PCR technique, whereas the histological and immunohistological analysis do not favor anymore a diagnosis of MF. Despite this fact, there was only an 18% recurrence rate after 6 months. This may suggest that the histological and immunohistological assessment of treatment results are better predictors of a persisting favorable treatment response than TCR rearrangement analysis.
The number of cases is too small to determine whether the intensity of adverse reactions and/or the post inflammatory hyperpigmentation after application are predictive of a favorable treatment outcome or not.
The adverse effects of IM were identical to those experienced using IM gel as AK therapy [1, 2]. In general, the adverse effects were less severe following the second IM application, probably linked to an already partial remission of the target lesion after the first IM application. Hyperpigmentation of the target lesion was frequent (8 out of 11 target lesions) but faded over time.
Final dosing regimens and the place of IM among the armamentarium of topical treatments against MF should be evaluated on larger series. Whether other ingenol derivatives are also effective as anti-MF topical agent remains to be determined [33]. One major drawback of IM is that it is only approved for a body surface limited to 25 cm2. Currently ingenol disoxate is being developed for use on larger surfaces, up to 250 cm2, as has been recently published for field AK [34].
CONCLUSION
In conclusion, this pilot study suggests that IM gel merits further evaluation as a topical alternative treatment for patients with localized patch/plaque stage MF skin lesions not exceeding 25 cm2, in a placebo-controlled study design. This study provides an initial proof of concept that IM also acts against neoplastic MF lymphocytes.
FINANCIAL-DISCLOSURE
Leo Pharma provided the IM gel but the authors perceived no financial compensation in what so ever form. Leo Pharma did not intervene in the design and interpretation of the study results.
ETHICAL APPROVAL AND CONSENT TO PARTICIPATE
This prospective pilot study was performed in accordance with the ethical standards of the University Hospital Committee on institutional human experimentation.
HUMAN AND ANIMAL RIGHTS
No Animals were used in this research. All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2008.
CONSENT FOR PUBLICATION
The aims of the study and all the procedures were explained to all the patients and all signed an informed consent (EudraCT number: 2017-000612-42).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


