All published articles of this journal are available on ScienceDirect.
0.1MG/ML Tamoxifen Gel Improves Plaque Psoriasis. An Open Study
DEAR EDITOR,
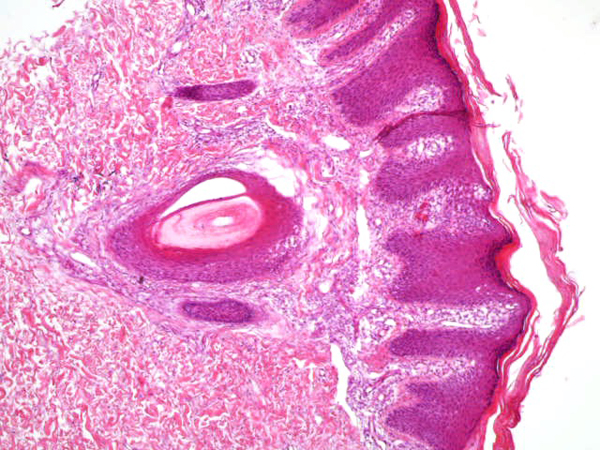
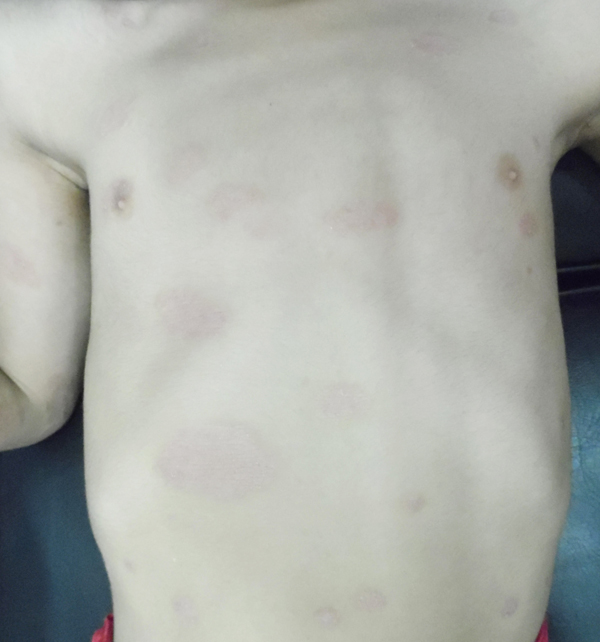
From September to October 2016, we have treated 6 patients affected by plaque psoriasis with 0.1mg/ml tamoxifen gel. The characteristics of the study population are summarized in Table 1. Mean PASI was 15.5. Each patient had undergone mainstay topical and systemic therapy with lack of a clinically significant response as per physician assessment (no decrease in PASI). No patient had systemic comorbidities, and the two female patients included were in menopause. In each case, psoriasis diagnosis had been confirmed by histological examination (Fig. 1).
Being a case series study, we obtained IRB waivers; all patients were required to sign an informed consent. Topical 0.1mg/ml tamoxifen gel in the liposomal base (Supplementary File S1) was applied twice/day to the affected skin for 15 days, with a dosage of 1 fingertip unit per 120 cm2 (half of one hand palm). All patients were followed up at 1 and 3 months; 2 patients reached the 5 months follow up. Tamoxifen and its metabolites (N-desmethyl tamoxifen; E and Z isoforms of 4-hydroxytamoxifen; N-desmethyl 4-hydroxytamoxifen) serum concentrations were assessed at 15 days of therapy in each patient. No other psoriasis treating drugs (topical or systemic) were administered during the study period.
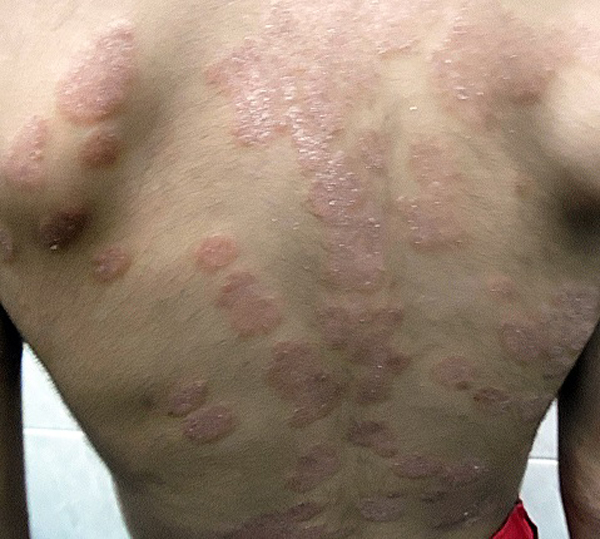
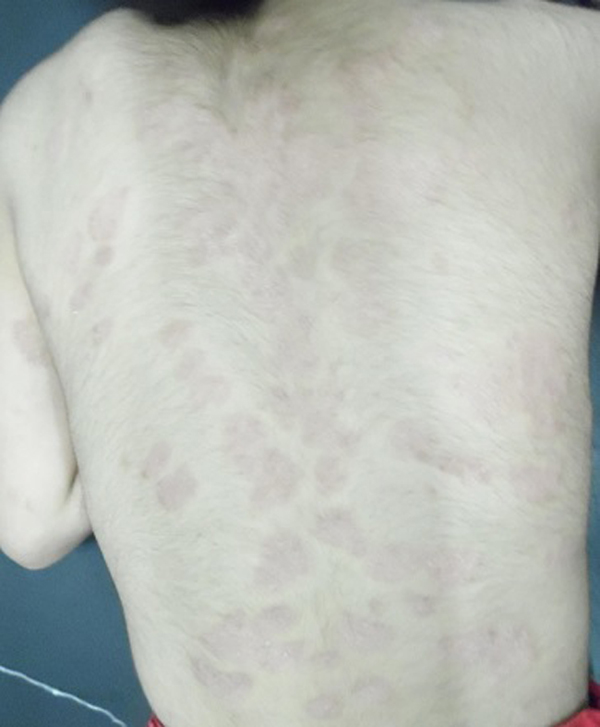
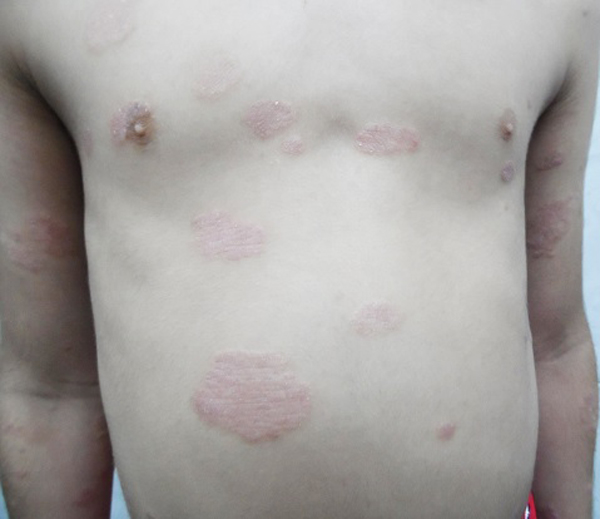
Mean PASI, as assessed by a blinded dermatologist after 15 days, 1 and 3 months were 7.5, 8 and 9.8 (Table 1), Figs. (2-5), respectively. The response was sufficiently sustained over time, with moderate signs of recurrence at 3 months. The two patients followed up for 5 months showed complete recurrence. No systemic or local adverse events were noted in any of the patients.
| – | Sex | Age | Psoriasis onset (years before) | Previous therapies * | Baseline PASI |
PASI at the end of the therapy (15 days) |
PASI at 1 month follow-up | PASI at 3 month follow-up | PASI at 5 month follow-up |
|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | M | 21 | 2 | 1, 2, 4 | 13 | 6 | 6 | 8 | – |
| Patient 2 | F | 53 | 15 | 1, 2, 4 | 15 | 5 | 6 | 8 | – |
| Patient 3 | M | 46 | 20 | 1, 2, 3 | 14 | 7 | 7 | 9 | 13 |
| Patient 4 | F | 56 | 24 | 1, 2, 5 | 20 | 10 | 12 | 14 | – |
| Patient 5 | M | 24 | 6 | 1, 2, 3, 4 | 18 | 10 | 10 | 12 | 15 |
| Patient 6 | M | 52 | 28 | 1, 2, 3, 5, 6 | 13 | 7 | 7 | 8 | – |
Sex hormone receptors are known to be present in the skin, as epidermal cells metabolize estrogen and progesterone, even if their specific effects are unclear [1]. Estrogen receptors are also present on T cells, especially in the CD8 subset, where they have been shown to decrease T-helper cell activity (CD4), as well as on vascular smooth muscle and endothelium [1]. These receptors further control the immune response through macrophages and thymocytes. The role of estrogens in psoriasis is complex, as they suppress the T-cell response, reduce cytokine production, stimulate keratinocytes proliferation and promote angiogenesis. Therefore, they may ultimately improve or worsen the dermatosis [1].
As an anti-estrogen, tamoxifen has been shown capable of inducing a shift from Th1 to Th2 immunity and exerts an anti-angiogenic action in different tissues [2]. Previous favorable response to oral administration of tamoxifen has been reported in two dated cases of plaque psoriasis [3, 4]. Recently, Bhatia et al. [5] used a mouse-tail model to evaluate the anti-psoriatic activity of topical tamoxifen encapsulated in the new generation phospholipid-based vesicular and micellar systems. The results of this study demonstrated the potential usefulness of topical tamoxifen in psoriasis.
In our observation, topical 0.1mg/ml tamoxifen gel was effective in a 15 days application regimen and provided a sufficiently sustained response up to 3 months in all patients, considering that just one 15 days trial was performed. While precise therapeutic protocols are yet to be defined, topical 0.1mg/ml tamoxifen may represent a novel option to manage hard-to-treat chronic plaque psoriasis; this may allow for an effective therapeutic response while avoiding the potential toxicity associated to conventional systemic therapies in patients showing an insufficient response to such treatments. To avoid complications from systemic absorption, we enrolled two female patients in menopause. As a matter of fact, tamoxifen serum metabolites were undetectable in all of our patients, indicating the safety of topical tamoxifen application, and reflected those of another experience in ductal carcinoma in situ of the breast [6]. However, the rate of absorption could, of course, vary based on the integrity and inflammatory status of the skin, as well as depending on the amount of gel used (some patients tend to apply more or less than prescribed). Therefore, at present, the definite conclusion regarding systemic absorption cannot be drawn. Further controlled studies are needed to confirm our observation and possibly to extend the therapeutic indication to other forms of psoriasis.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The author obtained IRB waiver for this study.
HUMAN AND ANIMAL RIGHTS
Not applicable.
CONSENT FOR PUBLICATION
All patients were required to sign an informed consent.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publishers Website along with the published article.


