All published articles of this journal are available on ScienceDirect.
Topical 1% Propranolol in Liposomal Gel: A New Adjuvant Tool for Chronic Leprosy Ulcers
Abstract
Objective:
To evaluate the effects of 1% topical propranolol in liposomal gel in 3 patients with plantar ulcers.
Methods:
We enrolled 3 patients with 3 ulcers who had completed the WHO recommended treatment regimen. The ulcers were cleaned with sterile normal saline, and 1% topical propranolol in liposomal gel was applied 2 times/day for 3 months, or less if complete healing was reached before. Assessment of ulcer re-epithelization was recorded at baseline, 6 weeks, and 3 and 6 months after initiation of treatment.
Results:
Response in the form of granulation tissue formation started by the second week. Substantial reduction in size subsequently continued over the next 3 months. Two of the 3 patients showed complete healing of the ulcers at the 6 months follow up. In the 3rd patient, the ulcer showed only modest signs of healing. Surprisingly, in all patients, the sensory function was restored, particularly in terms of pain. Some motor functional recovery at the ulcer site and surrounding tissue was also documented.
Conclusion:
To the best of our knowledge, this is the first trial of topical propranolol for the treatment of trophic ulcers of leprosy. This may represent a promising adjuvant therapy for leprosy ulcers, including ulcers of older age. Further studies are warranted with a larger number of patients and a longer period of follow up to determine the ideal candidates and to identify clinical factors predictive of response.
1. INTRODUCTION
Leprosy is a chronic infectious disease caused by a close relative of Mycobacterium tuberculosis, Mycobacterium leprae (M. leprae) [1]. Non-healing chronic trophic foot ulcers are a major problem and a major cause of handicap in patients with leprosy. Approximately 30% of patients with leprosy develop nerve damage. The involvement of peripheral nerves in the extremities in leprosy often results in a trophic ulcer. Trophic, or neuropathic, ulcer is a common complication of an anesthetic foot. The term plantar, trophic, or perforating ulcer was introduced in 1959. It was defined as a chronic ulceration of the anesthetic foot, situated in well-defined areas overlying bony prominences, resistant to local and/or systemic therapy, and characterized by a marked tendency to recur. The majority of neuropathic ulcers occur on the plantar surface of the feet, with approximately 70% on the forefoot. These ulcers are responsible for much of the morbidity associated with leprosy [2] and carry the potential of malignant transformation if recalcitrant, including squamous cell carcinoma and melanoma [3]. The chronicity of the ulcer is perpetuated by repeated inadvertent trauma or injury. Sensory loss, muscular paralysis, autonomic nerve damage, scar tissue formation, primary vascular insufficiency, and/or the direct action of M. leprae have been implicated in ulcer formation [4]. Despite relentless endeavors, neuropathic ulcers associated with leprosy continue to represent a treatment challenge. Conventional treatment of these wounds can be slow because of their chronic inflammatory state and the senescence of local reparative cells. Among the conventional treatment options, intralesional platelet rich-plasma [5] and topical phenytoin sodium zinc oxide paste have been shown to be successful [6]. Recently, three of the authors have tried, for the first time, topical β blocker therapy in the form of 1% propranolol under occlusion for a chronic plantar ulcer in an elderly man. Significant healing of the ulcers was noted 3 weeks later, with no side effects or recurrence at the 1-year follow up [7]. Herein, we report our application of 1% topical propranolol in liposomal gel for the treatment of chronic neuropathic ulcer of leprosy.
2. METHODS
We enrolled 3 patients with 3 ulcers (1 multi-neural leprosy and 2 borderline lepromatous variants) who had completed the WHO recommended treatment regimen and were in the follow-up period. All patients were thoroughly informed about the treatment and signed a written informed consent. All were undergoing a regular debridement of necrotic tissue and dressing of the ulcers. The patients had no comorbidities, except patient 3 who was also affected by type 2 diabetes. No systemic or intralesional therapies were given before or during the study. Bacterial cultures before and after treatment and radiography were performed in each case, with no evidence of active infection or findings of osteomyelitis. The morphological features of the ulcers were recorded, including size, site, depth, and presence of secondary infection. The ulcer was cleaned with sterile normal saline, and 1% topical propranolol in liposomal gel was applied 2 times/day for 3 months, or less if complete healing was reached before the end of 3 months. 1% propranolol liposomal gel was obtained using a reverse phase evaporation method, warranting a high encapsulation efficiency, up to 65%. The quantity of the drug used was directly proportional to the size of the ulcer (1 fingertip unit per 3 cm2) to achieve a uniform application across ulcers of different sizes. The patients were instructed to apply the gel at the margin and the base of the ulcer with gentle massaging followed by protective dressing. The patients were instructed to avoid excessive walking and to wear suitable shoes made for the deformed leprosy foot. Regular follow up was arranged biweekly to ensure patients’ adherence. Assessment of ulcer re-epithelization was recorded at baseline, 6 weeks, and 3 and 6 months after initiation of treatment (Table 1).
|
Patient (Sex) |
Duration of the Ulcers | Type of Leprosy | Comorbidities | Age | Ulcer Shape | Ulcer Size at Baseline | Ulcer Size at 6 Weeks | Ulcer Size at 3 Months | Ulcer Size at 6 Months | Bone Involvement | Sensitivity |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Case 1 (Male) |
10 ys | Multi-neural | None | 65 | Figurate | 5*4.6*0.5 cm | 1*1.5*0.5 cm | healed | healed | No | Improved |
| Case 2 (Male) |
8 ys | Borderline lepromatous | None | 62 | Figurate | 4.3*3*0.5 cm | 1*0.5*0.3 cm | healed | healed | No | Improved |
| Case 3 (Female) |
7 ys | Borderline lepromatous | Type 2 DM, stage IV melanoma |
57 | Figurate | 10*4.5*0.5 cm | 8*4*0.3 cm | 7*4*0.2 cm | 8*5*0.2 cm | No | Improved |
3. RESULTS
The response in the form of granulation tissue formation started by the second week. Substantial reduction in size subsequently continued over the next 3 months. Two of the 3 patients showed complete healing of the ulcers at the end of the study, while 1 patient showed only minor improvement (Figs. 1, 2, 3). Surprisingly, in all patients, good sensory function, up to 40%, was restored at the ulcers and in the surrounding tissue, particularly in terms of pain relief and reacquired sensation on the sole of the foot (tibial nerve sensitive fibers); some motor function, up to 25%, was also recovered, in terms of inversion and plantar flexation (tibial nerve motor fibers). None of the patients developed local or systemic adverse effects or relapsed to the pre-treatment state at 6-month follow up.
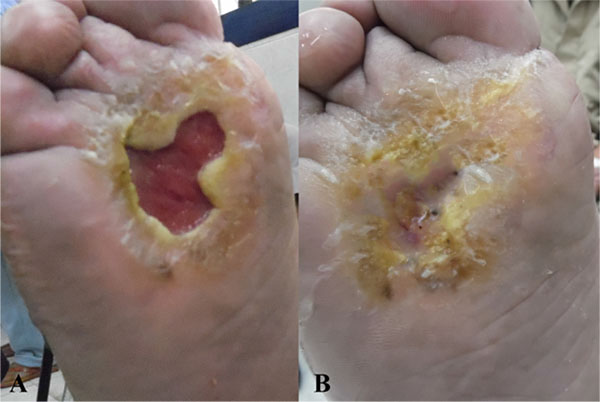
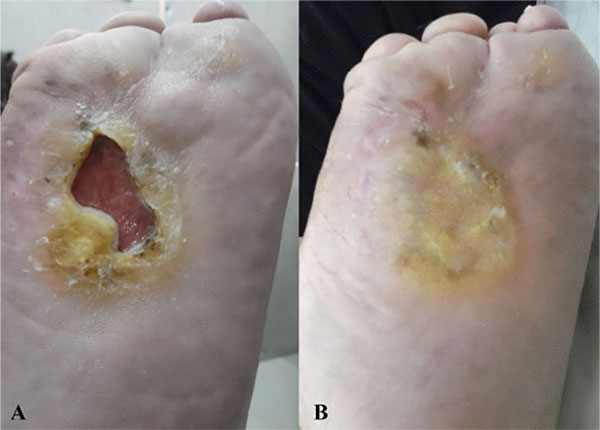
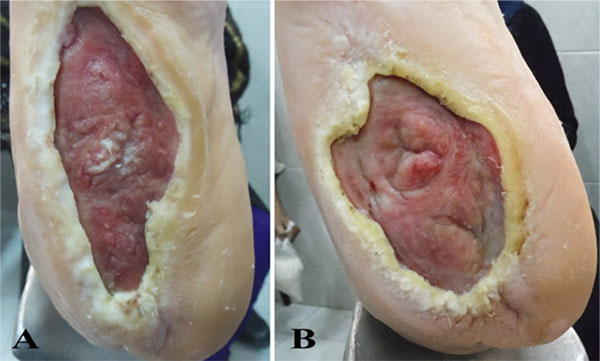
4. DISCUSSION
β-adrenergic receptors are present on keratinocytes, fibroblasts, and melanocytes. High levels of circulating catecholamines, associated with burns or traumatic wounds, are known to impair wound healing. Catecholamines are produced endogenously by wounded keratinocytes. Propranolol has been shown to block the negative effect that catecholamines exert on wound healing [8]. Furthermore, it may enhance keratinocyte migration [9] and angiogenesis in wounds, regardless of the specific etiology [10-13]. Intriguingly, infection of Schwann cells with M. leprae results in demyelination, axonal dysfunction, and immunological granulomatous reaction in the nerve [14]. In vitro studies demonstrated the ability of M. leprae to induce demyelination. M. leprae interacts with ErbB2 receptors on the surface of myelinating Schwann cells and causes myelinated Schwann cells to dedifferentiation into an unmyelinated cell via an internal signaling process [15-17]. Recently, Sysa-Shah et al. [18] identified a novel activation loop in the heart (and in vitro systems) linking ErbB2 and β-adrenergic systems, where β-adrenergic receptor stimulation causes elevation and activation of ErbB2. Notably, antigen presenting Langerhans Cells (LC) are anatomically associated with peripheral nerves. Some neuropeptides have been shown to regulate LC antigen-presenting function. Pretreatment of epidermal cells with epinephrine or norepinephrine in vitro suppressed the ability of these cells to present antigen for elicitation of delayed-type hypersensitivity in previously immunized mice [19]. Given the know proprieties of beta-blockers in wound healing in general, we hypothesized that 1% topical propranolol not only counteracts the inhibitory effect of catecholamines on tissue reepithelization, but also the local inhibitory effect on M. leprae-presenting Langerhans cells. Further, it may interrupt linkage of the ErbB2 and β-adrenergic systems, preventing Schwann cell demyelination, and subsequently preventing further trophic ulceration. To our knowledge, this is the first trial of topical propranolol in trophic ulcers of leprosy. Though complete healing of the ulcers was not reached in all cases, the drug may be a potential adjuvant therapy for leprosy ulcers, even for those of older age. We can only speculate why patient 3 did not seem to respond to topical propranolol. Certainly, her comorbidity might have played a role, as type 2 diabetes is known to impair wound healing, even when controlled by medication therapy. However, definite data is lacking. Moreover, based on our experience in wound healing using topical β blockers [7, 11-13], we noticed that leprosy ulcers require more time to initiate a response and to complete healing when compared to other types of chronic wounds [12]. Further studies are warranted including a larger number of patients and a longer period of follow up to determine the ideal candidates and to identify clinical factors predictive of response.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The local Ethical committee waived approval for this clinical study.
HUMAN AND ANIMAL RIGHTS
No Animals were used in this research. All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
A written informed consent was obtained from all patients when they were enrolled.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


