All published articles of this journal are available on ScienceDirect.
Fractional Carbon-Dioxide Laser Plus Topical Clotrimazole versus Oral Itraconazole plus Topical Clotrimazole for Onychomycosis: A Randomized, Controlled Trial
Abstract
Background:
Treating onychomycosis is problematic for a variety of reasons. The very nature of the hard, protective nail plate itself makes it difficult for topical drugs to reach the fungal pathogens beneath it. Oral therapy is more effective than topical therapy, but it is expensive, requires monitoring for toxicity, and can result in multiple drug interactions.
Objectives:
To compare the efficacy and safety of fractional CO2 laser combined with topical clotrimazole to oral itraconazole plus topical clotrimazole in the treatment of onychomycosis.
Methods:
A sample of 88 adults (between the ages of 18 and 78) was randomly divided into two groups. 45 patients received fractional CO2 laser therapy at an interval of 2 weeks and twice-daily application of clotrimazole 1% cream. 43 patients were treated by pulsed itraconazole (200 mg twice daily, 1 week on, 3 weeks off) and twice-daily application of clotrimazole 1% cream. The duration of the treatment was 3 months for fingernails and 4 months for toenails in both groups. The clinical effect was measured using the Scoring Clinical Index for Onychomycosis (SCIO index), KOH examination for the affected nails were performed, and liver function tests in the two groups were analyzed.
Results:
73% of patients treated with fractional ablative CO2 laser achieved a negative KOH examination compared with 79% of the itraconazole group (P>0.05). The SCIO reduction in the laser group was superior to that in the itraconazole group (P<0.001). Notably, a biochemical abnormality was not documented in patients who received laser treatment. In contrast, liver transaminases elevations without clinical symptoms were documented in two patients at the end of itraconazole therapy.
Conclusion:
Fractional CO2 laser plus a topical antifungal drug might be more clinically effective in the treatment of onychomycosis than itraconazole, without any adverse reactions. It could be an alternative for clinicians in onychomycosis cases in which oral antifungal agents are contraindicated.
1. INTRODUCTION
Onychomycosis is a fungal nail infection either caused by a dermatophyte (tinea unguium) or by other non-dermatophyte filamentous fungi or Candida spp [1]. Onychomycosis is a common condition accounting for about 50% of all nail problems [2, 3] with a worldwide prevalence of 5.5% (based on a weighted average of 6 epidemiologic studies published in 2016) [4-9]. The prevalence increases with age [10]. Onychomycosis is a cosmetically disfiguring and debilitating disease adversely affecting the self-esteem of patients and sometimes leading to depression. Apart from the pain and difficulty in walking, it carries the risk of cellulitis in the elderly and diabetic patients [11].
Treating onychomycosis is problematic for a variety of reasons. Oral therapy is more effective than topical therapy, but it is expensive, requires monitoring for toxicity, and can result in multiple drug interactions. Topical therapy is a long process that often requires nail debridement and multiple return visits and still delivers a relatively poor success rate. The very nature of the hard, protective nail plate itself makes it difficult for topical drugs to reach the fungal pathogens beneath it [12, 13]. Surgical nail avulsion for the treatment of onychomycosis should be avoided as potential side effects include post-operative pain, narrowing of the nail bed, distal paronychia, and infection [14]. It is contraindicated for patients with diabetes mellitus, peripheral and collagen vascular diseases, autoimmune disorders, disorders of hemostasis, and paronychia. Moreover, recurrences and reinfection are not uncommon (up to 20% of cured patients) [10]. Long-term negative effects on quality of life, difficult management associated with a high prevalence of onychomycosis show the need for new treatment modalities.
Recently, there has been a growing interest in the laser industry following a series of scientific trials that have introduced various laser systems as a therapeutic alternative for this condition. Lasers are potential therapeutic modalities for onychomycosis because they have very few contraindications, minimal adverse effects, short treatment regimens, and good patient compliance [15]. The CO2 laser system was the first laser device studied for onychomycosis. Tissue heating from laser machines can produce direct damage or induce an immune reaction against pathogens [16-18]. Furthermore, they have been studied as adjuvants to topical therapies for onychomycosis, as they can facilitate drug delivery through the nail plate by increasing permeability [19]. Ablative standard CO2 in combination with a topical antifungal drug, proved to be efficacious [20, 21]. However, patients experienced pain for weeks after treatment [22]. Yang et al. [23] used the ablative fractional CO2 laser for onychomycosis patients for 12 weeks with 8 sessions. The results showed clinical efficacy in 52%. Moreover, two other studies used fractional CO2 laser plus a topical medication (amorolfine and terbinafine, respectively) for onychomycosis treatment. The clinical efficacy was 71% and 73%, respectively [24, 25]. These results suggested that a combination of ablative fractional CO2 with topical antifungals could be more effective than fractional CO2 therapy alone. However, no randomized trial comparing this treatment approach to standard systemic antifungal therapy.
Therefore, we designed a randomized, controlled study to compare the safety and efficacy of carbon dioxide laser-assisted delivery of topical clotrimazole with systemic itraconazole plus topical clotrimazole in the treatment of onychomycosis.
2. MATERIALS AND METHOD
2.1. Patients
This study was performed at Thu Duc General Hospital, Ho Chi Minh, Vietnam, between September 2018 and June 2019. The study was conducted with approval from the Thu Duc General Hospital ethics committee. The study protocol conformed to the guidelines of the Declaration of Helsinki. Written informed consent was obtained from all study participants before enrolment. As part of the consent process, clinical study participants were informed about the risks and benefits of participating in the study.
Adults (aged ≥ 18 years) who were capable of giving written informed consent and who had a clinical diagnosis of onychomycosis were eligible. Suspected onychomycosis was confirmed by positive direct microscopy in all patients.
Patients were excluded if they were pregnant, lactating or had other nail diseases or history of systemic antifungal treatment within the last 6 months.
The participants’ clinical history was reviewed in detail, including age, sex, duration of disease, first anatomic site affected at the onset of the disease, drug intake, and complete physical examination.
2.2. Study Design
Patients were randomized into two groups (laser group and itraconazole group) using a computer randomization program.
Patients in the laser group were treated with fractional CO2 laser therapy (Lutronic, Korea) at an interval of 2 weeks with deep mode, pulse energy of 10 to 15 mJ, a density 10%, a pulse interval of 0.5 ms, and pulse width of 0.5 to 1.0 ms. The fluence was adjusted according to the thickness of the nail.
Patients in the itraconazole group received pulse itraconazole (200 mg twice per day, 1 week each month).
All subjects in the two groups received topical clotrimazole 1% cream twice daily. The duration of the treatment was 3 months for fingernails and 4 months for toenails in both groups.
2.3. Outcome Assessment
Direct examination with KOH was performed at the beginning and the end of the study. The results were counted as positive when specimens from the nail contained septated hyphae.
Clinical improvement, the Scoring Clinical Index for Onychomycosis (SCIO index), was evaluated by two experienced dermatologists before and after treatment. SCIO (range 1-30) was calculated using the clinical index component and the growth component in the following equation [26]:
Where d = depth of involvement, f = clinical form, h = degree of hyperkeratosis, l = location, and a = age of patients.
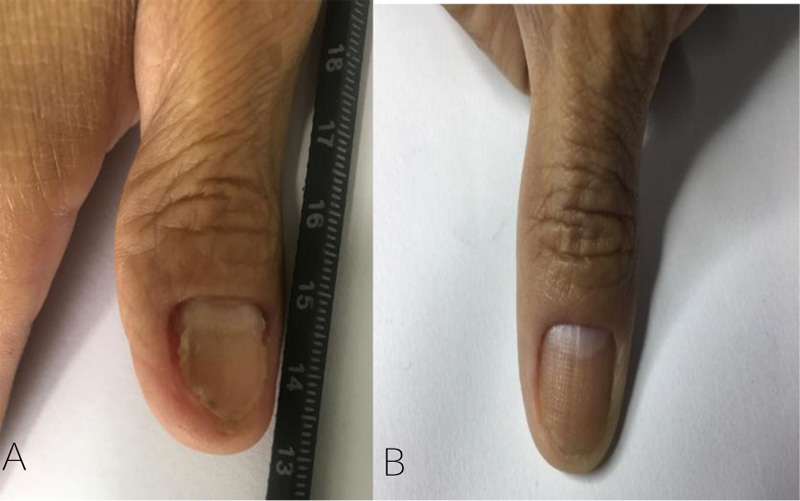
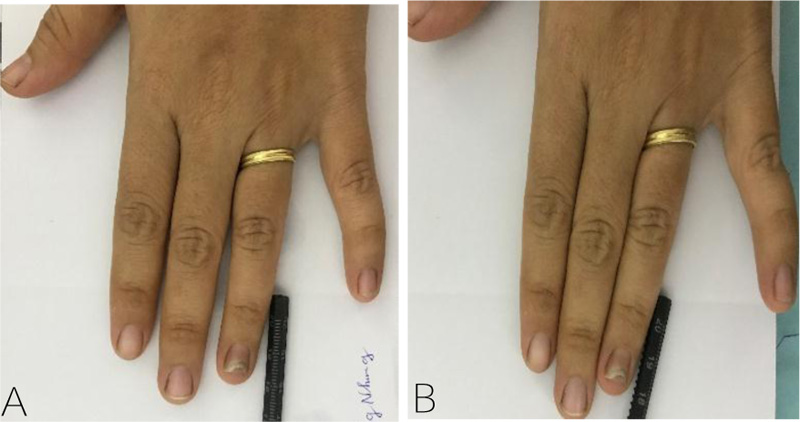
Digital photography documentation of nails was taken at baseline and every visit. At the last visit, the patient's satisfaction with the treatment results was evaluated by a questionnaire using a grading system as follows: very satisfied, satisfied, slightly satisfied, and dissatisfied.
For the safety evaluation, we determined whether the subjects had any subjective symptoms or objective findings. Liver transaminases were performed before, and at the end of the therapy, other investigations were performed only if clinically demanded.
2.4. Statistical Analysis
Statistical analysis was carried out using SPSS version 24.0 for Windows (IBM, Armonk, NY, U.S.A.). All quantitative variables were expressed using measures of central tendency (mean, median) and measures of dispersion (SD). The normality of the data was checked using the Kolmogorov–Smirnov test. Comparisons were made using the Student t-test for normal distributions, and the Mann– Whitney test for non-parametric variables. Qualitative or categorical variables were described as frequencies and proportions. Differences in categorical variables were compared using the chi-square test. All statistical tests were two-sided and performed at a significance level of p < 0.05
3. RESULTS
3.1. Demographics and Baseline Characteristics
In total, 88 patients were randomized and included in the study. 45 participants received fractional CO2 laser therapy plus topical clotrimazole and 43 patients received oral itraconazole in combination with topical clotrimazole. Patient demographics and baseline characteristics are shown in Table 1. The comparison of gender, mean age, and mean disease duration at the first visit between the two groups showed no significant difference (p < 0.05).
| - | Laser (n=45) | Itraconazole (n=43) |
|---|---|---|
| Sex N (%) | ||
| Male | 19 (42.2) | 16 (37.2) |
| Female | 26 (57.8) | 27 (62.8) |
| Age, y mean (95% CI) | 46.5 (42.8 - 50.2) | 48.3 (44.6 - 51.9) |
| Nail involvement N (%) | ||
| Toenail | 139 (72) | 77 (60.6) |
| Fingernail | 54 (28) | 50 (39.4) |
| Duration of onychomycosis, months mean (95% CI) | 14.4 (8.8 - 20) | 12 (8.5 - 15.5) |
| History of oral therapy N (%) | 14 (31.1) | 7 (16.3) |
3.2. Mycological Cure
At the end of the treatment period, direct microscopy was negative in 34 patients (79.1%) in the itraconazole group compared to 33 patients (73.3%) in the laser group (Table 2). This difference was not statistically significant (P=0.53).
3.3. Clinical Improvement
Among patients treated with fractional CO2 laser, the baseline mean SCIO was 7.5 (95% CI 5.4 to 9.6) and the mean SCIO decreased to 2.4 (95% CI 0.7 to 4.1) at the end of the treatment. For itraconazole treated patients, the baseline mean SCIO was 5.9 (95% CI 3.9 to 7.9) and decreased to 3.9 (95% CI 1.9 to 5.9) at the last visit. At the end of the study, both groups had statistically significant reductions of mean SCIO compared with the first visit (both p<0.01) (Fig. 1).
| - | Negative | Positive | p-value |
|---|---|---|---|
| Laser | 33 (73.3%) | 12 (26.7%) | 0.53 |
| Itraconazole | 34 (79.1%) | 9 (20.9%) |
Furthermore, the difference between the mean SCIO at baseline and the end of the study was compared between the two groups. Results showed that the reductions in the mean SCIO in the fractional CO2 laser-treated group was significantly greater than in the itraconazole-treated group (p<0.001) (Fig. 2).
3.4. Safety and Tolerability
Side effects were seen in 8 of 43 participants in the itraconazole- treated group. Side effects in the itraconazole-treated group were gastrointestinal effects (three patients), abdominal pain (one) headache (two), and asymptomatic self-limited liver transaminases elevations (two). In contrast, several participants experienced a slight pain with CO2 fractional therapy. No other adverse reactions were noted in the laser-treated group.
4. DISCUSSION
Up-to-date oral and topical antifungal agents (used alone or in combination) are considered the mainstay of therapy against onychomycosis. Even in the most experienced hands, these approved and widely used drugs may offer only disappointing results with high rates of relapse. Laser treatments are the most rapidly expanding area of onychomycosis therapy. Laser devices can be used to enhance drug delivery, activate topically applied drugs, or photothermally kill fungi. Laser treatments have a number of advantages over traditional therapy as they are primarily conducted in the clinic by trained professionals, reducing the need for patient compliance. They also have the potential to reduce adverse events and systemic interactions. To our knowledge, this study is the first controlled trial that compares the efficacy and safety of carbon dioxide laser-assisted delivery of topical clotrimazole to systemic itraconazole for onychomycosis treatment.
In the present study, there was more female than male patients (60% versus 40%). Similar results were found by previous studies [7, 9, 27, 28]. The difference might result from daily habits such as nail cosmetics, frequent water contact, detergents, raw food, cleaning materials, hydrocarbons, and trauma. The habit of sharing nail care equipment such as scissors, clippers, and emery boards in nail salons also contributes to the spread of infection [29-32]
In our study, onychomycosis involving toenails is about two times more common than that of fingernails. These findings are comparable to the results of a multicenter survey of 15,000 patients in Canada [3]. The warm, moist, confined environment provided by our occlusive footwear, combined with slower toenail than fingernail growth and less blood flow to the area, all combine to make the feet and toes specifically vulnerable.
Distal and lateral subungual onychomycosis was the most common pattern of onychomycosis in our study, followed by total dystrophic onychomycosis. A similar result was found in the studies of Gupta et al. [3], the largest epidemiology survey has reported.
We found no significant differences between laser and itraconazole group in the mycological clearance rate defined by negative KOH examination at the end of the study (73.3% vs. 79.1%).
However, the laser group showed a significantly higher clinical improvement defined as a reduction in the mean SCIO at the end of the study compared with the itraconazole group (P<0.001).
A few uncontrolled studies with a combination of fractional CO2 laser and a topical antifungal drug have been done. Lim et al. [24] was the first group using the combination of fractional CO2 laser and a topical antifungal agent for the management of onychomycosis. They treated 119 nails in 24 patients with fractional CO2 laser and topical amorolfine. The fluence used was at 24 J/cm2. The laser treatment consisted of three sessions at four-week intervals. Ninety-two percent of the patients showed a clinical response and 50% showed a complete response with a negative KOH. Similarly, Bhatta et al. [25] used fractional CO2 laser (also three sessions at four-week intervals) combined with daily 1% terbinafine cream for the treatment of onychomycosis. The authors reported 95% negative KOH and 92% negative culture at the end of the study. Most laser-treated nails (98%) showed improvement: the mean SCIO was significantly decreased.
To date, the exact mechanism of action of laser devices against fungal pathogens is still under investigation. Fungal inactivation through unselective tissue heating is the most prevailing hypothesis. It has been reported that temperatures of 55°C are the lowest capable of destroying dermatophytes [16], whereas temperatures of less than 45°C can stimulate germination with Trichophyton spp.-specific optimal values [17]. Tissue heating can produce direct damage or induce an immune reaction against pathogens. The optimal temperature and duration of heating tolerated by human tissue have not been elucidated yet. Nevertheless, heating should be done cautiously as temperatures above 60°C, which lead to protein denaturation and coagulation of healthy surrounding tissue. Carney et al. [18] showed that heat produced a fungicidal effect for T. rubrum at 50°C with exposure times of 15 min, for Epidermophyton floccosum at 50°C with exposure times of 10 min, and limited growth of Scytalidium spp. was seen at 55°C with exposure times of 5 min. Additionally, the fractional CO2 laser can create multiple tiny columns that facilitate drug delivery through the nail plate by increasing permeability [24]. Furthermore, during the laser procedure, nail plates, the environment for fungal growth, are partially vaporized [33].
In total, 11 adverse events related to itraconazole were experienced by 8 patients. The most frequently reported itraconazole-related adverse events were gastrointestinal disorders. Patients treated by fractional CO2 laser only experienced slight pain. This correlates with most published studies [24, 25, 34, 35].
CONCLUSION
Fractional CO2 laser combined with topical clotrimazole 1% is a safe and effective treatment option for onychomycosis. It achieved a greater clinical improvement with a similar mycotic cure rate rather than oral itraconazole in combination with topical clotrimazole 1%. Candidates for this method are patients who have failed previous therapies or those in whom systemic therapies are contraindicated, fearing polypharmacy, or drug interactions. Patients with certain non-dermatophyte molds, such as N. dimidiatum infections, in whom there is no good alternative therapy, may also be considered ideal candidates.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was conducted with approval from the ethics committee of the Thu Duc General Hospital, Vietnam under approval no. 62.72.35.01.
HUMAN AND ANIMAL RIGHTS
No Animals were used in this research. All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
Informed written consent was taken from all the patients when they were enrolled.
STANDARDS OF REPORTING
CONSORT guidelines have been followed.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


