SYSTEMATIC REVIEW
Topical 5% Imiquimod as Monotherapy for Primary Cutaneous and Cutaneous Metastatic Melanoma: Systematic Review of the Literature
Keegan O’Hern1, Meagan Chambers1, Shu T. Liang1, Dylan J. Badin1, Michael S. Chapman1, 2, *
Article Information
Identifiers and Pagination:
Year: 2021Volume: 15
First Page: 1
Last Page: 10
Publisher ID: TODJ-15-1
DOI: 10.2174/1874372202115010001
Article History:
Received Date: 9/9/2020Revision Received Date: 14/11/2020
Acceptance Date: 5/12/2020
Electronic publication date: 16/02/2021
Collection year: 2021

open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background:
Surgery is the gold standard treatment for primary cutaneous melanoma but may not be suitable given some comorbiditiies, lesion size or location, or anticipated functional impairment. Imiquimod (IMQ) is a topical immunotherapy infrequently used for melanoma, often in combination with other treatments.
Objective:
The present work aims to review the available literature on the safety and efficacy of imiquimod in the treatment of cutaneous primary and metastatic melanoma.
Methods:
We systematically reviewed the literature on topical imiquimod as monotherapy for melanoma, excluding in situ disease. MEDLINE, EMBASE, and CINAHL searches were conducted using terms related to imiquimod and melanoma, results summarized according to the PRISMA Guidelines and quality of evidence assessed using the GRADE tool.
Results:
Of 559 citations identified, 14 case reports and series with 38 patients with 95 lesions met inclusion criteria. There was heterogeneity in treatment regimens, including the number of applications and treatment length. Complete clearance was observed in 39% of cases, while the stable or progressive disease was seen in 42% of cases; treatment efficacy was limited in cases with prior metastatic disease.
Conclusion:
The current literature for the use of imiquimod in cutaneous primary and metastatic melanoma remains scarce, with most evidence derived from case reports and series likely to be influenced by selection bias for positive treatment results. Nevertheless, imiquimod remains a relatively well-tolerated treatment for cutaneous primary and metastatic melanoma that may be used in selected cases.
1. INTRODUCTION
Topical medications are increasingly utilized for skin cancer with a growing evidence base for safety and efficacy. Imiquimod (IMQ), a topical immunomodulator, has been FDA approved for the treatment of superficial basal cell carcinoma, genital and perianal warts, and actinic keratosis [1]. Its off-label, second-line use in melanoma in-situ has gained popularity when surgical management is complex or contraindicated due to proximity to important anatomic structures, large lesion size or number requiring extensive reconstruction, patient age, or comorbidities. There is uncertain but growing literature describing the use of topical IMQ for primary cutaneous melanoma and cutaneous metastatic melanoma. However, as IMQ is more commonly used in combination with other adjuvant therapies–including IL-2, topical retinoids, laser therapy, chemotherapy, and cryosurgery–its effectiveness as monotherapy remains unclear [2-5]. The aim of this literature review is to critically assess the efficacy and safety of IMQ as monotherapy in the topical treatment for primary cutaneous melanoma and cutaneous melanoma metastases.
2. MATERIALS AND METHODS
2.1. Literature Search Strategy and Study Selection
This systematic review was prepared in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and the Cochrane Handbook for Systematic Reviews of Interventions [6, 7]. A comprehensive search of the literature was built using keywords related to imiquimod and melanoma (Appendix). The search was run on November 11, 2019, in MEDLINE, EMBASE and CINAHL, yielding a total of 559 citations. Titles and abstracts, as well as full texts, were screened independently by two authors, with the resolution of any conflicts by a third author. References of included articles and relevant reviews were checked for additional articles missed by the initial search.
Studies that were included in this review described patients with primary cutaneous and cutaneous metastatic melanoma treated with imiquimod 5% cream monotherapy treatment; this included lentigo maligna melanoma, “classic melanoma,” and/or “disseminated” disease, but did not include melanoma in-situ (Table 1). Conference abstracts and case reports were included if they were peer-reviewed. Literature reviews, in vitro studies, and animal studies were excluded. Studies were also excluded if they were not in English or if they used imiquimod in combination with other topical or systemic therapies, including surgery. Individual patients from studies were excluded from our analysis if they received other concurrent therapies while being treated with imiquimod.
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Disease: Primary invasive melanoma and/or cutaneous metastatic melanoma (in transit and/or satellite metastases). Intervention: Topical application of 5% imiquimod cream as monotherapy. Design: Peer reviewed studies, including conference abstracts and case reports. |
Disease: Melanoma in-situ (and associated non-invasive variants). Non-melanocytic lesions. Intervention: Studies using imiquimod in combination with other topical or systemic therapies, including surgery. Design: Literature reviews, in vitro studies, and animal studies. Non-English studies. |
2.2. Data Collection Process and Risk of Bias Assessment
Data extraction and reconciliation were done independently by two authors, with disagreements resolved by a third reviewer. Structured data collection forms captured the following variables: article identifier (year, first author), type of publication (full text, abstract, or poster), study period, study design, number of cases (patients), diagnoses, age (mean and range), sex, number of lesions, location of the lesion(s), size of the lesion(s), type of intervention, number of treatment applications, treatment frequency, treatment duration, inflammation, adverse effects, pauses in treatment, clinical clearance, the performance of biopsy or other post-treatment assessment (e.g. Wood’s lamp examination, confocal microscopy, dermoscopy), histopathologic clearance, length of follow up, and recurrences.
Clinical clearance was defined as no residual pigmentation, while partial clinical clearance was defined as residual pigmentation on clinical examination, dermoscopy, confocal microscopy, or Wood’s lamp examination. The absence of clinical clearance was operationalized as no change in clinical appearance after completing an imiquimod regimen. Histological clearance was defined as the absence of residual melanoma in a biopsy or excision specimen obtained after finishing imiquimod treatment. The partial histological response was defined as histological clearance of at least 1 lesion with 1 or more residual lesions after treatment with imiquimod. The absence of histological clearance was defined as an unchanged clinical appearance with the presence of atypical melanocytes in a biopsy or excision specimen. Recurrence was defined as the clinical or histological presence of melanoma after previous complete clinical or histological clearance. Patients who did not complete treatment due to excessive inflammation or other reasons were considered to have failed treatment.
All studies meeting criteria were evaluated by two reviewers for methodological rigor and risk of bias using the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) tool [8-10]. A 13-point checklist was used, with one point awarded for each criterion: 0-3 was considered poor quality, while 4-7 was considered fair quality, 8-10 was defined as good quality, and 11-13 was defined as excellent quality. Studies that scored as “poor” quality were excluded.
2.3. Statistical Analysis
Patient characteristics, treatment regimens, and lengths of follow-up were analyzed using descriptive statistics. We pooled the data available on clinical clearance, histological clearance, treatment duration, and recurrence rates and expressed these results as means with ranges as appropriate. A formal meta-analysis could not be performed given the heterogeneity of studies included.
3. RESULTS
3.1. Study Selection
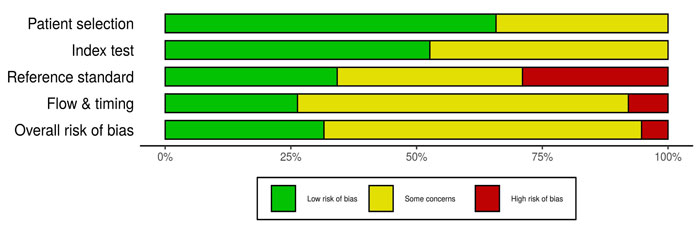
Details of the selection process for eligible studies are shown in Fig. (1). Of 559 citations generated from the literature search, 14 articles met inclusion criteria (Table 2) [11-24]. All studies included were case reports or case series. There were minimal overlapping data reported in two articles [15, 17]; all other duplicate data sets were removed. The GRADE risk of bias assessment tool demonstrated a moderate risk of bias among included studies (Fig. 2). Only 5 of 14 included studies reported negative outcomes.
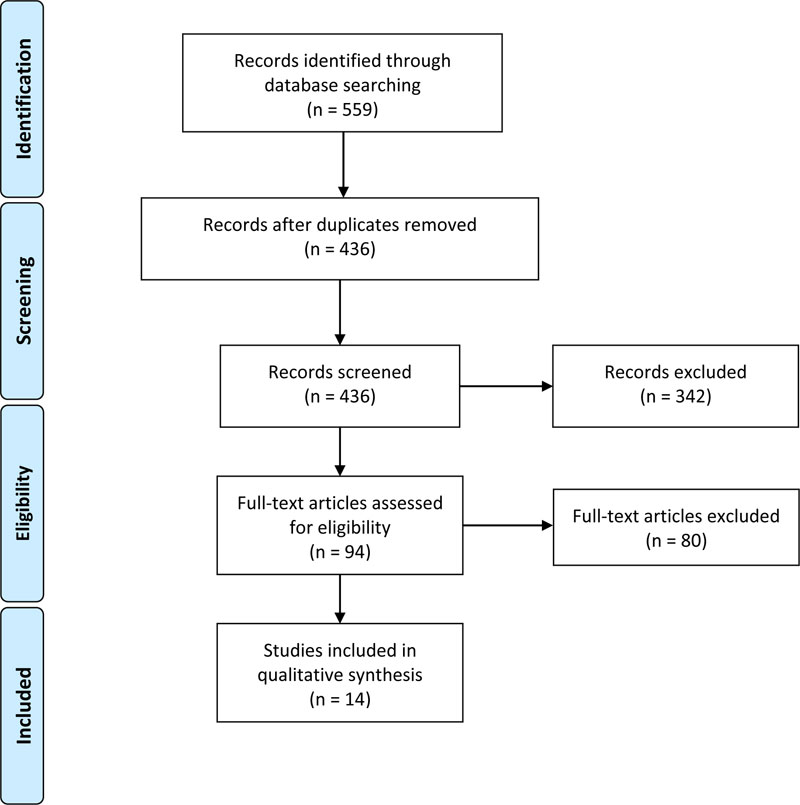 |
Fig. (1). PRISMA Flow Chart. Details of the selection process for eligible studies. |
| Author(s), Year of Publication | Patient Gender/ Age | Primary Tumor | Prior Treatments | # with Prior Metastatic Disease | Lesion (#, location) | Treated Lesion Size and Staging | Treatment Protocol | Results and Follow-up | Adverse Effects |
|---|---|---|---|---|---|---|---|---|---|
| Steinmann et al. 2000 [11] | 6 patients, unspecified gender and age | AJCC III/IV (from 2000) | NR | NR | Multiple, NR | Stage III/IV (AJCC) cutaneous melanoma metastases | 12+ total applications of IMQ (3/wk, ≥4 wks), epifocal application | Complete clinical clearance with biopsy confirmation: 1 patient; Remained stable: 2 patients; Recurred/progressed: 3. Post treatment follow-up: 12-36 wks | After an unspecified time, local skin irritation |
| Ugurel et al. 2002 [12] | 1 male, 81 | NR | Surgical removal of primary 6 months prior to multiple metastases/IMQ | 1 | Multiple, upper right arm | 6 cm area of multiple satellite metastases | 56 total applications of IMQ (7/wk, 4 wks followed by 3.5/wk, 8 wks), with occlusion. Area of application: 2 cm around 6 cm lesion | Double-check biopsy info: Complete clearance in all cutaneous metastases) but regional lymph node metastases occurred during IMQ treatment at week 12. Biopsy confirmation and ultrasound of draining LNs. Post treatment follow-up: NR | After 4 weeks, erythema, erosive patches. After 6 weeks (2 weeks after switching to every other day application), inflammation decreased. After 10 weeks, moderate signs of inflammation |
| Bong et al. 2002 [13] | 1 female, 82 | Breslow 3mm Clark IV, positive SLNB, superficially spreading malignant melanoma | Excision of the primary tumor, inguinal LN dissection (d/t positive sentinel LN bx), immunochemotherapy with dacarbazine 850 mg/m^2 i.v. q4 wks and IFN alpha 3x3 mio. IU s.c. per wk for 6 mo. All of this happened in the 16 mo. before she had the new amelanotic dermal metastases | 1 | ≥15, right thigh | Amelanotic dermal metastases, cutaneous metastases | 140 total applications of IMQ (5/wk, 18 wks), with occlusion | Partial clearance, with biopsy confirmation. 1 remaining lesion was excised. Post treatment follow-up: NR | After 1 week, mild flu-like symptoms. After 2 weeks, superficial erosions and moderate erythema. After a few more weeks, moderate tolerable pruritus |
| 1 male, 74 | Breslow 2.5 mm Clark IV, superficially spreading malignant melanoma, inguinal LNs involved | The same dose of dacarbazine, IFN-alpha as above, but also retrograde venous perfusion of liposomal doxorubicin 6 times | 1 | ≥15, left lateral knee | Amelanotic dermal metastases, cutaneous metastases | 140-252 total applications of IMQ (14/wk then reduced to 5/wk, 28 wks), with occlusion | Partial clearance, with biopsy confirmation. 1 remaining lesion was excised. Post treatment follow-up: NR | Initially, intense inflammation-like reaction. Over the entire treatment period, strong erythema and erosions | |
| Vereecken et al. 2003 [14] | 1 female, 67 | In transit metastases of melanoma | Multiple surgical excision of prior metastases, LN dissection, isolated limb perfusion with melphalan and TNF | 1 | 1, left lower leg (initially diagnosed at 57) | Locoregional relapse, cutaneous achromic metastases | 40 total applications of IMQ (5/wk, 8 wks), with occlusion | Complete clearance, with biopsy confirmation. Post-treatment follow-up: 0 wks | After an unspecified time, mild irritation |
| Wolf et al. 2003 [15] | 1 female, 86 | Breslow 1.9 mm Clark IV. No LN or visceral metastases on staging CXR and US of abdomen/LNs | Wide excision of primary melanoma (1999), CO2 laser ablation of several skin metastases 1 (Aug 2000), excision of 2 of multiple new metastases (Dec 2000), the rest of which treated w/ IMQ | 1 | Multiple, right lower leg | Multiple across the lower half of shin | 48 total applications of IMQ (3/wk, 16 weeks). Application 1 cm around lesion | Complete clearance, with biopsy confirmation. Post treatment follow-up: 60 wks | After unspecified time, mild peritumoral erythema |
| 1 male, 49 | Breslow 1.8 mm Clark IV. Original sentinel node was positive with micro-metastases, radical LN dissection of neck done. No other systemic disease. | Wide excision of primary melanoma (2/2000), Radical neck LN dissection after + SNLB, low dose interferon-alpha 2b (3 million IU 3 times per wk for 3 mo.), systemic chemo (dacarbazine, carboplatin, fotemustine); followed by excision, CO2 laser vaporation and regional hyperthermia combined with x-ray treatment | 1 | Multiple, retro auricular | Multiple coalesce into unspecified suprauricular plaque (~6-7 cm), other micro metastases sprinkled posteriorly spanning another ~5 cm vertically | 96 total applications of IMQ (3/wk, 32 weeks). Application 1cm around lesion | Complete clearance, with biopsy confirmation. Post-treatment follow-up: 32 wks | After 1 month, erosions were seen but resolved after discontinuation | |
| Hesling et al. 2003 [16] | 1 female, 77 | No spread to LNs or viscera detected prior to IMQ treatment (just multiple local cutaneous metastases | Superficial spreading melanoma (thickness unknown) on chest resected in 1989 (age 65 yo at the time), in 1995 (age 71 yo) pagetoid (superficial) recurrences completely excised. In 2000 (76 yo) | 1 | Multiple, chest and right axillary | Local cutaneous metastases | 56 total applications of IMQ (7/wk, 8 wks) | 90% of the lesions had clinical clearance. Post-treatment follow-up: 104 wks, died of metastatic melanoma | NR |
| Wolf et al. 2004 [17] | 1 female, 86; 5 unspecified | Locoregional cutaneous metastases of malignant melanoma | NR | NR | Multiple, right lower leg | Locoregional cutaneous metastases | 48 total applications of IMQ (3/wk, 16 wks). Application 1 cm around lesion. Other patients are NR treatment length | Complete clinical clearance with biopsy confirmation: 2 patients; Partial clearance: 1 patient; No regression: 1 | NR |
| Wolf et al. 2007 [18] | 1 male, 77 | NR | Prior surgical excision of melanoma metastases before the appearance of multiple satellite metastases treated in this study | 1 | Multiple, forehead | Multiple satellite metastases | 35 total applications of IMQ (7/wk, 5 wks) | Complete clinical clearance, with post-treatment biopsy at 5-16 weeks post treatment. Post treatment follow-up: 27 wks | After 2-4 weeks, severe irritation of treated areas occurred, producing an extensive erythema with erosions |
| Alomar et al. 2007 [19] | 1 patient, unspecified age and gender | NR | NR | 0 | 1, face | LMM | Several months of treatment | Complete clearance, with biopsy confirmation. Post-treatment follow-up: 72 wks | After several months, inflammatory response was seen |
| Heber et al. 2009 [20] | 1 male, 63 | Breslow 1.4 mm Clark IV, Stage IIIC ulcerated, lymphatic involvement, and satellite metastasis. Surgically resected | Surgical removal of primary tumor 10 weeks prior to recurrence and imiquimod | 0 | 45, right parietal occipital | 0.7 cm, Subcutaneous and cutaneous metastases | 340 total applications of IMQ (5/wk, 68 wks) | 45 lesions complete clinical clearance. Post treatment follow-up: 16 wks | After an unspecified time, moderate erythema, erosions, mild itching/burning |
| Turza et al. 2010 [21] | 1 female, 59; 5 unspecified | Breslow 0.75 mm Clark III, superficially spreading, regression with multiple metastases locally and subcutaneous nodules | Wide local excision of primary superficial spreading melanoma in Jan. 2003. In March 2004, multiple metastases in 4x5cm region anterior to previous excision were treated with wide local excision. Margins were clear at the time. August 2004 enrolled in melanoma peptide trial (UVA MEL-43) for adjuvant therapy, but presented with metastatic lesions on scalp so withdrew from trial. Sept 2004 to May 2005 multiple recurrences, not surgical resected due to prior recurrences. Began high dose IL-2, temozolamide, another melanoma peptide vaccine trial UVA MEL-41, but progressed at the scalp. Then offered IMQ | 6 | Multiple, scalp | 4 x 5 cm cutaneous metastases | ~120 total applications of IMQ (5/wk, 24 wks). 3 patients did not follow protocol | Complete clinical clearance with biopsy confirmation: 2 patients; Recurred/progressed: 3 (including 1 patient who the authors did not explicitly declare recurrence, but is stated to have had a poorer response than the other patients who recurred/progressed). Post-treatment follow-up: NR | NR |
| Salerno et al. 2012 [22] | 9 patients, unspecified age and gender | Cutaneous in-transit metastases | NR | 9 | Multiple, NR | Cutaneous in transit melanoma metastases | NR | Complete clinical clearance: 2 patients; Partial clearance: 3 patients; Recurred/progressed: 3. Post treatment follow-up: NR | NR |
| Zattra et al. 2012 [23] | 1 female, 42 | Breslow 0.8 mm Clark III with large in situ component | NR | 0 | 1, back | 1.4 x 1.5 cm amelanotic melanoma Breslow's 0.53 mm Clark III with large in situ component | NR | No response with biopsy confirmation. Superficial spreading achromic malignant melanoma with large in situ component post treatment. Post treatment follow-up: 12 wks, wide excision performed | NR |
| Verga et al. 2019 [24] | 1 female, 77 | Breslow 0.8 mm, staging CT was negative for metastatic deposit, surrounding area of in situ disease with superficial spreading malignant melanoma | No | 0 | 1, malleolar area | 6.7 x 5.5 cm invasive primary melanoma of ankle Breslow's 0.8 mm with in-situ surrounding | 72 total applications of IMQ (13.4 wks, ~4wk pause at day 41 due to florid erythema). Application directly on lesion | Complete clearance, with biopsy confirmation. Post treatment follow-up: ~208 wks | After 4.6 weeks, florid erythema. After 5.4 weeks, superficial necrosis, thick scab. After 1.4 weeks of recontinuing treatment, less intense reaction that lasted ~2 weeks |
3.2. Summary Statistics
The 14 included studies are summarized in Table 2. Publication year ranged from 2000 - 2019. In total, there were 38 patients treated with imiquimod for primary cutaneous melanoma and cutaneous metastatic melanoma (Table 3).
There were a variety of melanocytic diagnoses, including three cases of primary cutaneous melanoma (invasive malignant melanoma, lentigo maligna melanoma, and amelanotic melanoma) as well as cutaneous melanoma metastases. The mean age was 71 years (range: 59-86 years) with an F:M ratio of 1:1.6 in the 11 studies reporting patient characteristics. A majority (n=35, 92%) of patients had American Joint Committee on Cancer (AJCC) stage III or higher disease at the time of treatment with imiquimod.
The number of melanoma lesions per study ranged from 1 to 45, with ≥95 total lesions across all included studies. Seven studies only reported qualitative descriptions of the lesions (such as “many”). Of the 13 patients whose lesion location was described, the majority of melanoma lesions were located on the legs (n = 5, 38%), followed by scalp (n = 4, 31%), torso (n = 2, 16%), face (n = 1, 8%), arm (n = 1, 8%).
Information about prior treatment was provided in 8 studies representing 10 patients. Surgical excision was performed in all 10 of these patients, chemotherapy in four patients, interferon-alpha in three patients, laser ablation in two patients, isolated limb perfusion with melphalan and TNF-alpha in one patient, and regional hyperthermia with radiation in one patient. In 12 studies reporting the reason why imiquimod was chosen in lieu of surgery, nine patients had failed prior treatment, four patients had a contraindication to surgery, three patients refused surgical intervention, and an incorrect diagnosis of amelanotic melanoma as basal cell carcinoma accounted for one case [23].
Across 11 studies reporting imiquimod treatment length, the reported treatment regimens ranged from 4-68 weeks with an average of 18.7 weeks. The frequency of imiquimod application ranged between 3-14 times per week, with an average of 5.7 applications per week. The total number of applications of imiquimod–calculated by multiplying the average number of weeks of treatment with the number of weekly imiquimod applications–ranged between 12-340, with an average of 101 applications per patient. Occlusion was utilized in 4 of 38 (10.5%) patients. Imiquimod application was performed with a 0-2 cm circumference around the lesion, as described in 5 studies.
3.3. Adverse Effects
Nine of the 14 included studies (64%) described inflammatory side effects associated with imiquimod treatment, including erythema in six (43%) studies, erosions in five (36%) studies, and mild irritation in three (21%) studies. It should be noted that these effects are expected. Systemic side effects, including flu-like symptoms, fever and headache, were noted in one patient. Florid inflammation and erosions necessitated a treatment hiatus after approximately four weeks in two patients (5.2%) for one to four weeks before restarting treatment.
3.4. Treatment Outcomes
All 14 included studies reported clinical outcomes of treatment with imiquimod. At least one patient with complete clinical clearance was reported in 11 of the 14 included studies, representing 15 of the 38 patients (39%); this includes 2 of 3 (66%) patients with primary cutaneous melanoma and 13 of 35 (37%) patients with cutaneous metastatic melanoma (Table 4). Seven patients with cutaneous metastatic melanoma (20%) had partial clinical clearance. Of the 38 included patients, 16 (42%) had the stable or progressive disease; patients who did not comply with the prescribed treatment protocol were included in the stable and/or progressive disease group for this analysis [21].
| Patient Characteristics | |
|---|---|
| Studies | 14 |
| Patients Primary melanoma Cutaneous metastases |
38 3 35 |
| Lesions | >=95 |
| Primary melanoma | 3 |
| Cutaneous metastases | >=92 |
| Male/Female/Unknown | 5/8/25 |
| Age | Mean: 71 (range: 59 - 86) |
| Location (by study) | - |
| Leg | 5 |
| Scalp | 4 |
| Trunk/Torso | 2 |
| Face | 1 |
| Arm | 1 |
| Treatment (total number of applications) | Mean: 101 (n=11 studies, range: 12 - 340) |
| Treatment (number of applications per week) | Mean: 5.7 (n=11 studies, range: 3 - 14) |
| Treatment duration (weeks) | Mean: 18.94 (range: 4 - 68) |
| Follow-up duration (weeks) | Mean: 52.3 (n=10 studies, range: 0 - 208) |
| Complete clinical clearance treatment (total number of applications) | Mean: 86.7 (range: 12 - 340) |
| Complete clinical clearance treatment (number of applications per week) | Mean: 4.4 (range: 3 - 7) |
| Complete clinical clearance treatment duration (weeks) | Mean: 19.8 (range: 4 - 68) |
| - | Clinical response reported: Total (n=14 studies, 38 patients) |
Clinical response reported: Primary melanoma (n=3 studies, 3 patients) |
Clinical response reported: Metastatic melanoma (n=11 studies, 35 patients) |
Histology after treatment: Total (n=11 studies, 17 patients) |
Histology after treatment: Primary melanoma (n= 3 studies, 3 patients) |
Histology after treatment: Metastatic melanoma (n= 8 studies, 14 patients) |
|---|---|---|---|---|---|---|
| Complete clearance | 15 (39%) | 2 (67%) | 13 (37%) | 11 (65%) | 2 (67%) | 9 (65%) |
| Partial clearance | 7 (19%) | 0 (0%) | 7 (20%) | 2 (12%) | 0 (0%) | 2 (14%) |
| Non-clearance | 16 (42%) | 1 (33%) | 15 (45%) | 4 (23%) | 1 (33%) | 3 (21%) |
After treatment with imiquimod, histopathological examination was performed on biopsy or excision specimens in 11 of the included studies totaling 17 patients. Pathologic clearance was observed in 11 of these 17 patients (65%) from nine of these 11 studies. Two of 14 (14%) patients with cutaneous metastatic melanoma showed partial pathologic clearance, and three patients (21%) showed no pathologic response to imiquimod.
Clinical follow up after treatment ranged from 0-208 weeks, with an average of 52.3 weeks across 10 studies reporting follow up. There were no recurrences observed during follow-up. If progression of disease occurred, it was identified at the cessation of treatment in all cases, with the exception of one case that demonstrated fatal metastatic spread 2 years after treatment cessation, though only 90% of lesions responded initially to treatment [16].
4. DISCUSSION
Overall, there is a paucity of evidence regarding the treatment of primary cutaneous melanoma and metastatic melanoma with topical IMQ monotherapy. No studies in this literature review directly compared IMQ to surgical treatment or other second-line therapies. Across the 14 studies and 38 patients included in this analysis, we identified a clinical clearance rate of 39% with IMQ as monotherapy for melanoma, which includes 2 of 3 (66%) patients with primary cutaneous melanoma and 13 of 35 (37%) patients with cutaneous metastatic melanoma. The histological clearance rate of the 17 patients who underwent a post-treatment biopsy was 65%, which included 2 of 3 patients (66%) with primary cutaneous melanoma and 9 of 14 (65%) patients with cutaneous metastatic melanoma. Of note, there was 100% concordance between clinical and histological clearance observed in 11 patients with post-treatment histological examination. The overall clearance rate identified in this study is strikingly lower than that previously reported by Sisti et al., who described a complete clinical clearance of 82.3% among included patients with cutaneous primary and metastatic melanoma treated with IMQ [25]. This may reflect the larger sample size (n = 38 vs. n = 17) and more rigorous inclusion and exclusion criteria in this review.
In scenarios where surgical excision and systemic treatment are prohibited due to patient or clinical factors, one must weigh the relative efficacy and safety of imiquimod versus alternative therapies. For primary cutaneous melanoma, definitive radiation therapy is not curative and thus may be used palliatively or adjuvantly for primary or cutaneous metastatic melanoma [26]. Non-resectable locoregional disease–including primary tumors, in-transit, satellite– may also be treated with isolated limb perfusion (ILP) or infusion (ILI) with melphalan in combination with tumor necrosis factor-alpha or other chemotherapies. As with imiquimod, there have not been any direct comparisons between ILP/ILI and surgical excision, but complete clearance rates for this treatment range from 22-50%, though with a much higher incidence of grade 3 or higher toxicity than imiquimod, ranging from 29-33% [27, 28]. Talimogene laherparepvec (T-VEC) oncolytic virus therapy was FDA-approved in 2015 for the treatment of unresectable advanced stage cutaneous or subcutaneous melanoma; however, not only is its overall response rate fairly low at 26.4%, the administration of this modality requires specialized centers experienced with oncolytic virus therapies [26, 29]. Efficacy data are limited for other therapeutic modalities such as laser and intralesional therapies (IL-2, BCG, etc.) [26]. Lastly, while systemic immunotherapies (anti-CTLA-4 and anti PD-1 therapies) hold great promise, their role in locoregional disease is evolving with ongoing clinical trials, and their cost remains prohibitive for some patients. Thus, while many questions persist regarding imiquimod’s efficacy and utility in treating melanocytic lesions, further research as both monotherapy and in conjunction with other modalities is warranted.
IMQ is a Toll-like receptor 7 (TLR7) agonist with potent antitumor effects mediated by activation of the innate and adaptive immune system. Following topical application, robust activation of plasmacytoid dendritic cells, Langerhans cells, and subsequent recruitment of inflammatory cells mediate a local immune response as evidenced by the erythema, irritation, and erosions experienced by patients during treatment [30].
This review aims to clarify the clinical effect of IMQ as monotherapy in cutaneous melanoma. The results demonstrate a clinical and histologic clearance rate well below that of prior estimates for patients receiving IMQ as part of combination therapy. Taken together, these findings highlight the augmentation of imiquimod’s efficacy that is likely seen when used adjuvantly. There were no recurrences among patients achieving complete clinical or histological clearance during follow-up after treatment cessation. However, new metastatic deposits were identified at the cessation of treatment in some cases. Among patients achieving clinical and/or histological clearance, the treatment regimen consisted of an average of 86.7 IMQ total applications (range 12-340 applications), with an average of 4.4 applications per week (range 3-7 per week) for a duration of 19.8 weeks (range 4-68 weeks).
Strengths of this literature review include the quality assessment of the included studies with the GRADE tool, as well as the rigorous methodology to identify cases with only IMQ treatment without other concurrent therapies that may obscure the true efficacy of this monotherapy. The overall limited sample size, heterogeneity of patients, variable treatment regimens, and lack of comparative study methodologies among the included studies were significant challenges limiting the generalizability of this review. With only 10 of 38 (26%) patients having prior treatments reported, it is difficult to contextualize how prior treatments may have impacted the efficacy of imiquimod. Moreover, the lack of common outcome measures and selection bias impeded a formal meta-analysis of this work. The reported treatment regimens varied considerably in duration and frequency, as did follow-up–ranging from 0-4 years with an average of only 52.3 weeks. This hinders the assessment of long-term efficacy given that melanoma typically recurs within two to three years after treatment, though there have been numerous reports of recurrence after 10 or more years after treatment [31]. Additionally, the available evidence is likely impacted by selection bias given the nature of case reports and case series to preferentially report positive treatment outcomes with imiquimod. While there are no randomized controlled trials to assess the efficacy of imiquimod in the treatment of melanoma, available evidence suggests a spontaneous complete regression rate of 0.22% to 0.27%, such that the estimated efficacy observed in this study is likely not due to chance alone [32].
The results of this review are of importance to dermatologists who require alternative treatment options for patients with primary cutaneous melanoma and cutaneous metastatic melanoma who have a contraindication or refuse surgery and/or systemic therapies. This is especially pertinent for elderly patients with comorbidities implicating excessive surgical risk, or for whom the cutaneous tumor metastasis burden is too great to be addressed with surgery alone.
CONCLUSION
We found an overall clearance rate of 39% for the treatment of primary cutaneous melanoma and cutaneous metastatic melanoma with IMQ monotherapy. The treatment of invasive primary or metastatic melanoma with IMQ is a potential salvage therapy for patients who are not candidates for, or decline, surgical/systemic management. There remains a dearth of evidence about this uncommon therapy, and while it may have some utility in clearing cutaneous metastases, larger studies with standardized treatment regimens and outcomes are needed.
ABBREVIATION
| Imiquimod | = IMQ |
CONSENT FOR PUBLICATION
Not applicable.
STANDARDS OF REPORTING
This systematic review was prepared in accordance with PRISMA guidelines.
FUNDING
None.
CONFLICT OF INTEREST
Dr. Chapman receives consulting funding from DUSA Pharmaceuticals, research funding from Biofrontera, Pfizer Inc., and Celgene Corporation for investigator-initiated clinical trials, and serves on the Genentech Inc. Advisory Board. All other authors have no disclosures or conflicts of interest.
ACKNOWLEDGEMENTS
Declared none.
APPENDIX
Search strategy (adapted for PubMed, Web of Science, and Embase):
Melanoma and Imiquimod or Aldera or zartra or Imiquimodum or Vyloma or zyclara.
REFERENCES
| [1] | FDA Imiquimod Prescribing Information [Internet]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/020723s022lbl.pdf |
| [2] | Green DS, Dalgleish AG, Belonwu N, Fischer MD, Bodman-Smith MD. Topical imiquimod and intralesional interleukin-2 increase activated lymphocytes and restore the Th1/Th2 balance in patients with metastatic melanoma. Br J Dermatol 2008; 159(3): 606-14. |
| [3] | Leventhal JS, Odell ID, Imaeda S, Maverakis E, King BA. Treatment of melanoma in-transit metastases with combination intralesional interleukin-2, topical imiquimod, and tretinoin 0.1% cream. JAAD Case Rep 2016; 2(2): 114-6. |
| [4] | Zeitouni NC, Dawson K, Cheney RT. Treatment of cutaneous metastatic melanoma with imiquimod 5% cream and the pulsed-dye laser. Br J Dermatol 2005; 152(2): 376-7. |
| [5] | Matas-Nadal C, Sòria X, García-de-la-Fuente MR, et al. Immunocryosurgery as monotherapy for lentigo maligna or combined with surgical excision for lentigo maligna melanoma. J Dermatol 2018; 45(5): 564-70. |
| [6] | Higgins J, Green S, Eds. Cochrane Handbook for Systematic Reviews of Interventions 1st ed. 2008. |
| [7] | Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med 2009; 6(7)e1000097 |
| [8] | Foroutan F, Guyatt G, Zuk V, et al. GRADE Guidelines 28: Use of GRADE for the assessment of evidence about prognostic factors: Rating certainty in identification of groups of patients with different absolute risks. J Clin Epidemiol 2020; 121: 62-70. |
| [9] | Yang AW, Li CG, Da Costa C, Allan G, Reece J, Xue CC. Assessing quality of case series studies: Development and validation of an instrument by herbal medicine CAM researchers. J Altern Complement Med 2009; 15(5): 513-22. |
| [10] | Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med 2009; 6(7)e1000100 |
| [11] | Steinmann A, Funk J, Berger T, Maczek C, Schuler G, von den Driesch P. First experiences with the topical immune response modifier imiquimod for the treatment of cutaneous melanoma metastases. J Invest Dermatol 2000; 115(3): 589-9. |
| [12] | Ugurel S, Wagner A, Pföhler C, Tilgen W, Reinhold U. Topical imiquimod eradicates skin metastases of malignant melanoma but fails to prevent rapid lymphogenous metastatic spread. Br J Dermatol 2002; 147(3): 621-4. |
| [13] | Bong AB, Bonnekoh B, Franke I, Schön M, Ulrich J, Gollnick H. Imiquimod, a topical immune response modifier, in the treatment of cutaneous metastases of malignant melanoma. Dermatology 2002; 205(2): 135-8. |
| [14] | Vereecken P, Mathieu A, Laporte M, et al. Management of cutaneous locoregional recurrences of melanoma: A new therapeutic perspective with imiquimod. Dermatology 2003; 206(3): 279-80. |
| [15] | Wolf IH, Smolle J, Binder B, Cerroni L, Richtig E, Kerl H. Topical imiquimod in the treatment of metastatic melanoma to skin. Arch Dermatol 2003; 139(3): 273-6. |
| [16] | Hesling C, D’Incan M, Mansard S, et al. In vivo and in situ modulation of the expression of genes involved in metastasis and angiogenesis in a patient treated with topical imiquimod for melanoma skin metastases Br J Dermatol 2004; 150(4): 761-7. |
| [17] | Wolf IH, Richtig E, Kopera D, Kerl H. Locoregional cutaneous metastases of malignant melanoma and their management. Dermatol Surg 2004; 30(2 Pt 2): 244-7. |
| [18] | Wolf IH, Kodama K, Cerroni L, Kerl H. Nature of inflammatory infiltrate in superficial cutaneous malignancies during topical imiquimod treatment. Am J Dermatopathol 2007; 29(3): 237-41. |
| [19] | Alomar A, Dalmau J. Lentigo maligna melanoma treated with Imiquimod. Arch Dermatol Res 2007; 299(5–6): 284-4. |
| [20] | Heber G, Helbig D, Pönitzsch I, Wetzig T, Harth W, Simon J-C. Complete remission of cutaneous and subcutaneous melanoma metastases of the scalp with imiquimod therapy. J Dtsch Dermatol Ges 2009; 7(6): 534-6. |
| [21] | Turza K, Dengel LT, Harris RC, et al. Effectiveness of imiquimod limited to dermal melanoma metastases, with simultaneous resistance of subcutaneous metastasis. J Cutan Pathol 2010; 37(1): 94-8. |
| [22] | Salerno EP, Wang E, Marincola F, Slingluff CL. Topical imiquimod induces immune activation and regressions of cutaneous melanoma metastases. J Immunother 2012; 35(9): 751-2. |
| [23] | Zattra E, Salmaso R, Tonin E, Alaibac M. Achromic superficial spreading melanoma accidentally treated with imiquimod. Acta Derm Venereol 2012; 92(1): 107-8. |
| [24] | Verga E, Chohan B, Verdolini R. Malignant melanoma treated with topical imiquimod: A bespoke treatment that spared the amputation. Case Rep Dermatol 2019; 11(1): 1-6. |
| [25] | Sisti A, Sisti G, Oranges CM. Topical treatment of melanoma skin metastases with imiquimod: A review. Dermatol Online J 2014; 21(2)13030/qt8rj4k7r6 |
| [26] | Michielin O, van Akkooi ACJ, Ascierto PA, Dummer R, Keilholz U. Cutaneous melanoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2019; 30(12): 1884-901. |
| [27] | Cornett WR, McCall LM, Petersen RP, et al. Randomized multicenter trial of hyperthermic isolated limb perfusion with melphalan alone compared with melphalan plus tumor necrosis factor: American College of Surgeons Oncology Group Trial Z0020. J Clin Oncol 2006; 24(25): 4196-201. |
| [28] | Dossett LA, Ben-Shabat I, Olofsson Bagge R, Zager JS. Clinical response and regional toxicity following isolated limb infusion compared with isolated limb perfusion for in-transit melanoma. Ann Surg Oncol 2016; 23(7): 2330-5. |
| [29] | Andtbacka RHI, Kaufman HL, Collichio F, et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol 2015; 33(25): 2780-8. |
| [30] | Singh M, Khong H, Dai Z, et al. Effective innate and adaptive antimelanoma immunity through localized TLR7/8 activation. JI 2014; 193(9): 4722-31. |
| [31] | Rueth NM, Cromwell KD, Cormier JN. Long-term follow-up for melanoma patients: Is there any evidence of a benefit? Surg Oncol Clin N Am 2015; 24(2): 359-77. |
| [32] | Emanuel PO, Mannion M, Phelps RG. Complete regression of primary malignant melanoma. Am J Dermatopathol 2008; 30(2): 178-81. |