All published articles of this journal are available on ScienceDirect.
Case Report: Successful Treatment of Giant Condyloma Acuminata with Intralesional Injection of Purified Protein Derivative
Abstract
Condyloma acuminata can be treated with different modalities, including topical and destructive procedures. However, treatment of large recalcitrant lesions tends to be difficult with the risk of pain, scarring and recurrence. Here we report a case of a large foul-smelling condyloma acuminata, treated successfully with intralesional purified protein derivative (PPD) injections.
1. INTRODUCTION
Warts are the most common cutaneous infection that is caused by the human papillomavirus (HPV) via direct or indirect contact. Condyloma acuminata, large cauliflower genital warts, are manifestations of anogenital human papillomavirus infections. They may be associated with pain, foul-smelling discharge and negative psychological effect. They spread through direct skin-to-skin contact, usually during oral, genital, or anal sex with an infected partner [1]. Treatments include topical creams, destructive methods, and immunotherapy. There is increased evidence for using intralesional immunotherapy for recalcitrant, recurrent, and extensive genital warts. It is non-destructive, easy to use and less painful [2]. Despite the fact that several vaccines have been used to clear warts, few studies assessed the effect of intralesional (IL) Purified Protein Derivative (PPD) in treating genital warts and hardly found any study or case report that assesses the effect of IL PPD injections in treating or debulking large condyloma acuminata. The advantages of PPD over other immunotherapy are its ubiquity, ease of access and low cost.
2. CASE
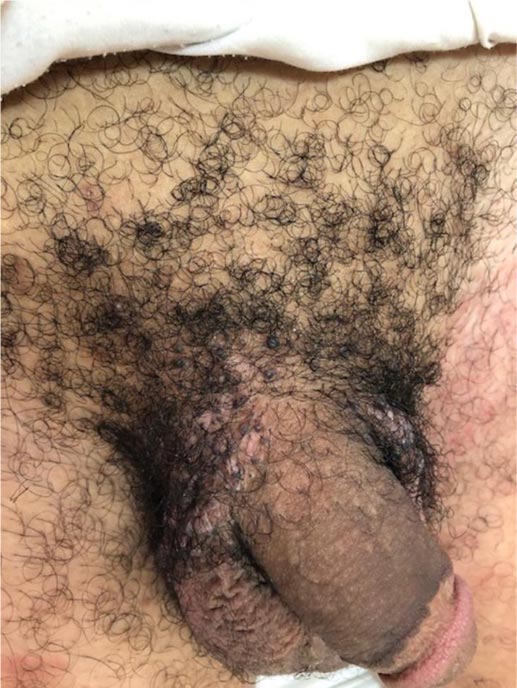
A 26-yr-old man with no significant past medical history presented with large multiple warty lesions on the pubic area for more than 5 months. It has been rapidly progressive and coalescing to form a giant mass with a warty surface. There was a history of multiple unprotected heterosexual encounters with multiple partners. Examination revealed multiple large warty lesions with foul-smelling discharge on the pubic area. There was no urethral discharge, genital ulcers or lymph nodes enlargement.
Routine blood investigations were normal and serological screening tests for HIV, HBV, HCV and syphilis were negative. In view of the size of the lesion, destructive treatment modalities were discussed, but the patient declined. Therefore, intralesional immunotherapy with Purified Protein Derivative (PPD) was administered in a biweekly dose of 0.2-0.3 ml distributed over two to three different sites, at least two centimeters apart. No topical or local anesthesia injections were needed as the patient felt very little discomfort with this procedure. More than 98% clearance was achieved after 7 sessions (Figs. 1 and 2). Subsequently, four sessions of cryotherapy and topical podophyllin were given to treat the remaining small few warty papules. The lesions were successfully eradicated with no recurrence ten months after the last visit (Fig. 3).


3. DISCUSSION
Condyloma acuminata refers to anogenital warts caused by the Human Papilloma Virus (HPV), which is the most common sexually transmitted infection. HPV6 and 11 are the most common strains that cause anogenital warts [3]. Genital warts are usually asymptomatic and can be found most commonly on the cervix, vagina, perineum, penis, scrotum and perianal skin. Most genital warts are seen in people between the age of 16 and 29 years which is similar to other sexually transmitted diseases like gonorrhea, syphilis and genital herpes simplex [4, 5].
Topical and systemic immunotherapies have now found a significant place in the treatment of warts because of their non-destructive nature, ease of use and promising results [2]. They are becoming more popular especially in the treatment of refractory cutaneous and genital warts. They act by enhancing or inducing the cell-mediated immune system to target and destroy the infected cells. Some of these agents are injected intralesional and include PPD, BCG vaccine, MMR vaccine, candida antigen and trichophyton antigen [6]. Intralesional immunotherapy can be used in different age groups, even in children, as Nofal and coworkers mentioned in their study [7].
Many studies have used IL PPD alone or alternating with other types of immunotherapy in treating different types of warts [8, 9]. It can be used as a valuable first-line treatment in difficult to treat sites like palmoplantar warts and periungual warts. In addition, immunostimulation with IL PPD provides increased chances of treating warts on distant sites and attaining a retained immune response for whole life [10, 11]. Riza and his colleagues found that immunostimulation with IL PPD is dose-dependent and multiple injections may be used for faster clearance [12].
Despite many studies that have been performed to detect the effect of immunotherapy in treating different types of warts, sparse reports exist on the effect of IL PPD in treating condyloma acuminata. In 2011, Eassa and his colleagues published a study showing that 85% of pregnant women with anogenital warts improved after receiving weekly intradermal PPD injections [2]. This study showed that PPD is safe in pregnancy compared to other immunotherapies like MMR. Another study was done in 2005 by Metawea, which showed a significant response of topical applications of BCG vaccine over condyloma acuminate [13]. Recently, Gupta published the first case of a female patient with extensive genital warts successfully treated with intralesional immunotherapy in the form of Bacillus Calmette–Guérin (BCG) vaccine [14].
CONCLUSION
In this case report, we show a case of giant condyloma acuminata treated successfully with intralesional PPD injections. More than 98% clearance was seen after 7 sessions of treatment. This promising option can be used as a primary treatment modality and should be considered before deciding to expose the patient to destructive methods or wide local surgical excision for giant condyloma acuminata.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the Forces Medical Services Ethics Committee under approval code FMC-EMC 001/2021.
HUMAN AND ANIMAL RIGHTS
No Animals were used in this research. All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
Informed consent was obtained from the patient involved in the study.
STANDARDS OF REPORTING
CARE guidelines have been followed.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of this study are available within the article.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


