All published articles of this journal are available on ScienceDirect.
Basal Cell Carcinoma Destruction by a Concentrate of Proteolytic Enzymes Enriched in Bromelain: A Preliminary Report
Abstract
Objectives:
Basal Cell Carcinoma (BCC) is the most common skin cancer generally treated by a variety of surgical and non-surgical destructive therapies. A Concentrate of Proteolytic Enzymes Enriched in Bromelain (CPEEB) derived from the stems of pineapples is approved for use for debriding deep burns. Prior studies suggest that bromelain also has anti-tumor effects. We describe our preliminary off-label treatment experience using topical CPEEB for the destruction of six BCCs in three patients.
Methods:
CPEEB was self-applied by three patients on six different Morphea, nodular, and superficially invasive BCCs. The CPEEB was applied as a thin layer prior to bedtime and left for a period of 9-12 hours. The wound was then covered with a petrolatum-based ointment for the next 24 hours. Application of the CPEEB was repeated up to 5 times over the course of 10 days, during which the patients were monitored daily and reevaluated by a board-certified plastic surgeon. If necessary, the CPEEB was reapplied up to five additional times over the next 10-day period. If necessary, any remaining lesion was surgically excised (MOHS surgery). The patients were then followed for up to 1 year.
Results:
Six BCCs located on the face, neck, and extremities were self-treated by three patients with 2-6 CPEEB applications. All of the BCCs were completely removed after the CPEEB application. CPEEB application was associated with local irritation and mild itching pain which resolved untreated within hours. In one patient, two of the lesion’s sites were surgically excised after 6 months with no tumor cells noted on histopathology. None of the BCCs recurred over the next 1 year.
Conclusion:
Our preliminary findings are a proof-of-concept that a concentrate of proteolytic enzymes enriched in bromelain may be a safe and effective destructive treatment for basal cell carcinomas. Future studies on larger groups of BCC patients are necessary in order to elucidate the potential use of CPEEB for this indication.
1. INTRODUCTION
Skin cancer is the most common cancer in humans, mostly caused by unrepaired solar-induced DNA damage. About 8 of 10 skin cancers are Basal Cell Carcinomas (BCC), with over ~4.3 million cases annually in the United States alone [1-4]. Treatment of BCC is mainly based on tumor destruction by a variety of surgical (e.g., excisional biopsy followed by MOHS micrographic surgery) and non-surgical (e.g., cryosurgery, electric or laser desiccation, 5-fluorouracil cream, imiquimod) methods. However, many of the current therapies require specialized personnel or facilities (e.g., MOHS surgery) or are prolonged (5-flurouracil), often involving adverse events. Furthermore, most topical therapies result in considerable local irritation and high recurrence rates [5].
A Concentrate of Proteolytic Enzymes Enriched in Bromelain (CPEEB), derived from the stems of pineapples, the Active Pharmaceutical Ingredient (API) used in this off-label treatment, is approved as a safe and effective debriding agent for deep burns in many parts of the world [6, 7]. It is also in an FDA-approved clinical trial for the debridement of chronic ulcers [8]. In this report, the same approved API was used off-label on a much-reduced area compared to burns or chronic wounds in three patients that could not be treated at the hospital during the COVID-19 lockdown. Wanting to avoid exposure to the pandemic as well as surgery, the patients asked to try this bromelain preparation that they already knew of. In addition to its proteolytic properties, a number of studies have demonstrated that bromelain has anti-tumor and inflammation modulation activities [9-18].
In a chemically (DMBA–TPA) induced mouse skin papilloma model, topical application of bromelain reduced tumor formation and tumor volume. It was shown in this study that bromelain inhibition was mediated through induction of p53, a shift in the Bax/Bcl-2 ratio, induction of caspases, decrease in Cox-2 expression, and inhibition of the NFκB pathway by regulating the MAPK and Akt/PKB pathways [12, 13]. Bromelain led to a reduction in proliferation of both human epidermoid carcinoma-A431 and melanoma-A375 cell lines and suppressed their potential for anchorage-independent growth [14]. Bromelain also inhibited metastasis-associated platelet aggregation and tumor cell invasiveness in the B16F10 murine melanoma cell line [15]. The pro-apoptotic effect of bromelain was further confirmed in oral squamous cell carcinoma (SCC) cell lines Ca9-22 and SCC25 [16]. In addition, bromelain is known for its inflammation modulation activity that may play a role in the healing of the treated sites [9, 17-20].
Based on the above, our hypothesis was that the proteolytic and anti-tumor activities of CPEEB would destroy the tumor mass, creating an ulcer at the site of the BCC. We also hypothesized that the CPEEB would abrade the skin surrounding the tumor, thus exerting its effect not only on the main bulk of the tumor but also on potential stray tumor cells at its periphery, preventing recurrence. It is our belief that the proposed mode of action and favorable safety profile of CPEEB in numerous pre-clinical and clinical studies would support the development of the product for the treatment of actinic lesions such as BCCs by the patient at home under the direct supervision of a skin cancer specialist using telemedicine. Herein, we describe our preliminary experience in treating BCCs in a small number of patients.
2. METHODS
We report our preliminary off-label treatment experience with three patients with six BCCs: Morphea type, superficial and nodular BCCs treated with CPEEB. The diagnosis of BCC was made clinically by a board-certified plastic surgeon with over 40 years of experience based on the clinical appearance of the lesions. Patients were informed that the CPEEB was off-label use of a drug approved for other cutaneous indications and that they might experience temporary irritation or itching pain after its topical application. They then signed a detailed informed consent form indicating that the use of the CPEEB was off-label.
The patients and their families were given a detailed explanation of the preparation and use of the CPEEB (that they already knew of for burns). The product was supplied as a dry powder of the API (0.5 gm) mixed with 5 ml of a hydrating vehicle immediately prior to its application. The active CPEEB gel was applied as a thin layer over the entire lesion together with a thin (2-5 mm) border of the normal surrounding skin and covered with an occlusive dressing and outer dressing to hold the CPEEB in place. The CPEEB was applied prior to bedtime and left on for a period of 9-12 hours. The dressing was then removed, and any remaining topical agent was washed off with soap and water. The wound was then covered with a petrolatum-based ointment for the following day. The patients were instructed to reapply the CPEEB up to five times over the course of the next 10 days. The patients photographed the lesion before and after each application of CPEEB and contacted the surgeon by telemedicine to determine whether or not to proceed using the CPEEB . The treatments were performed by the patients at their homes and monitored by telemedicine as it took place during the COVID-19 pandemic and local lockdowns.
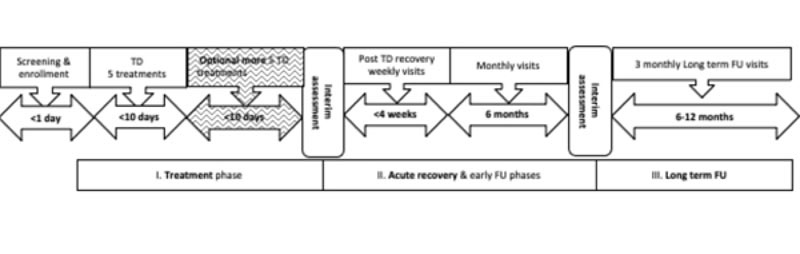
After the 10-day period, the patients were seen by the surgeon, who recommended either discontinuing the treatment if complete destruction of the BCC was achieved, or continuing to reapply the CPEEB up to another five times over the course of the next 10 days. At the end of this treatment period, the patients were seen by the surgeon, and a determination was made whether to proceed to the next phase of wound healing or perform an excisional biopsy followed by MOHS histology. Fig. (1) is a schematic representation of the treatment plan.
After completion of the destructive phase, the resulting wound was treated with a petrolatum-based ointment for the next 3-4 weeks until the wound healed. A low potency topical corticosteroid was used for several days towards the end of the wound healing phase to prevent the formation of granulation tissue. The patients were then followed up every 2-3 months for a period of up to 1 year to determine any recurrence of the tumors.
3. RESULTS
We treated six individual, 2-3 years old BCCs that occurred on three fair-skinned (types 1 and 2 on Fitzpatrick skin color scale) patients. A summary of the lesions and treatments is presented in Table 1. The first patient was a 37-year-old female with one Morphea type and two superficially invasive BCCs (Figs. 2-4). The second patient was a 72-year-old male with two nodular BCCs (Figs. 5 and 6). The third patient was a 70-year-old female with one superficially invasive BCC (Fig. 7).
| Patient no. and site of BCC | Lesion type | No. of CPEEB applications | Total exposure time (hrs.) | Results |
|---|---|---|---|---|
| Patient 1, left cheek | Morphea BCC | 5 | <60 | Complete TD + MOHS |
| Patient 1, right posterior neck | Superficial BCC | 5 | <60 | Complete TD + MOHS |
| Patient 1, left forearm | Superficial BCC | 5 | <60 | Complete TD |
| Patient 2, left arm | Nodular BCC | 6 | <72 | Complete TD |
| Patient 2, left leg | Nodular BCC | 5 | <60 | Complete TD |
| Patient 3, left shoulder/arm | Morphea BCC | 5 | <60 | Complete TD |
All patients complained of temporary skin irritation and mild itching pain that generally started around 1 hour after application and resolved untreated over the next 2-4 hours. The pain intensity at its worse was rated as 3-5 on an 11-point verbal numeric scale from 0 (none) to 10 (worst imaginable). No analgesic agent was needed throughout the entire treatment course. All patients were generally satisfied with the topical treatment especially compared to previous experiences with surgery and 5FU treatment.
All abraded ‘normal’ skin that surrounded the original borders of the BCC healed in about one week. All of the ulcerated lesions that remained after tumor destruction, some small full-thickness ulcers, healed within 2-4 weeks. None of the lesions resulted in hypertrophic scars. There were no clinical recurrences of tumors in any of the patients over the 12-month follow-up period (Figs. 2-7).
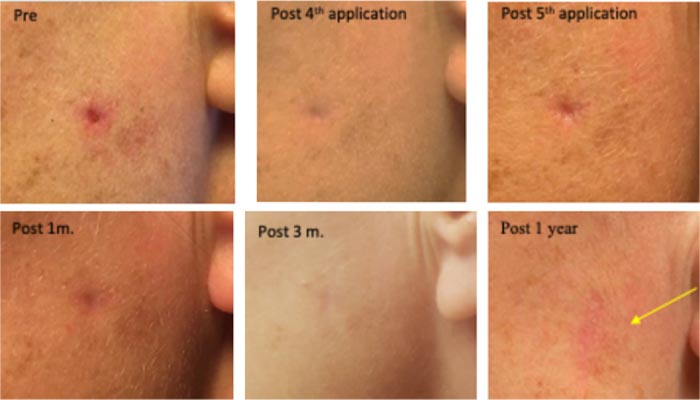
On her left cheek, a clinically diagnosed invasive Morphea type BCC (existing >3 years) with a central deep crater, causing an irritating pricking sensation. Treated five times, in 9-12 hr. sessions with CPEEB. Some irritation was noted during the first 3 applications. Clinical tumor destruction was assessed after the 5th application, and CPEEB applications were stopped. Recovery and healing of the abraded skin with filling of the full-thickness defect of the lesion center were slow, lasting 7 weeks. At 3 months, a shallow, depressed scar can still be seen. Wide margin MOHS surgery of the treated site at 6 months demonstrated no residual tumor cells. At 1 year, the surgical scar can be seen (Fig. 2).
Treated 5 times in 9-12 hr. sessions, with CPEEB. Some irritation was noted during ~1 hour after the first 3 applications. Clinical tumor destruction was assessed after the 5th application, and CPEEB applications were stopped. Recovery and healing of the abraded skin and lesion bed took 1 week. At 1 month, a mildly depressed scar is seen, and at 3 months, the flat scar can barely be seen. Wide margin MOHS surgery of the treated area at 6 months demonstrated no residual tumor cells. At one year, the surgical scar can be seen (Fig. 3).
Treated 5 times in 9-12 hr. sessions with CPEEB. Some irritation during ~1 hour post first 2 applications. Clinical tumor destruction was assessed after the 5th application, and CPEEB applications stopped. Recovery and healing of the abraded skin and lesion bed took 1 week. At 1 month, a fine scar formed, and at 3 months and 1 year, a flat scar is barely seen (Fig. 4).
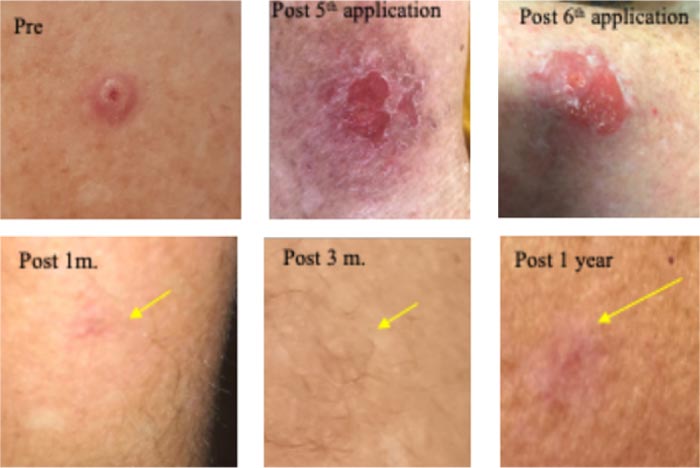
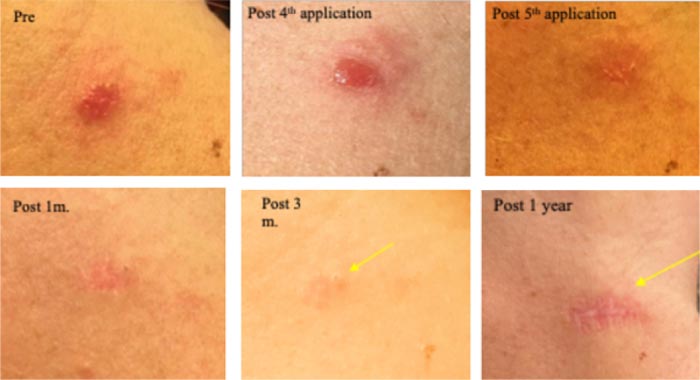
A large 6mm lesion left arm appeared 3 years ago, causing a pricking, irritating sensation. Clinically diagnosed as a large nodular BCC. Received five, 9-12 h sessions with CPEEB. Some irritation during ~1 hour after the first 2 applications. Clinical tumor destruction was assessed after the 5th application, but the tumor “footprint” could be seen (arrow) before and after wiping the lesions, so a 6th CPEEB application was performed, eliminating this “footprint”. After a 1-week recovery, the surrounding irritation is clearing with a central, small, 3 mm. deep crater that took 2 additional weeks to heal. Recovery continued, and after 1 month, a fine scar was formed, and at 3 months and 1 year, a flat scar is barely seen (Fig. 5).

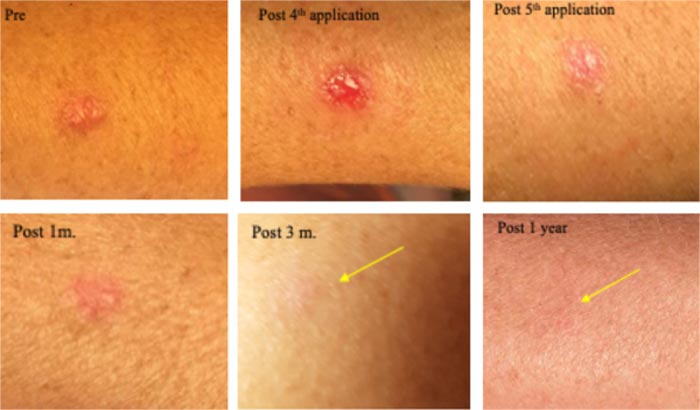
Clinically diagnosed as a large nodular BCC. Treated 5 times, 9-12 h sessions with CPEEB. Some irritation was noted during ~1 hour after the first 3 applications. Clinical tumor destruction was assessed after the 4th and 5th CPEEB applications. After 6 weeks, it healed completely, and at 3 months and 1 year, a flat scar is barely seen (Fig. 6).
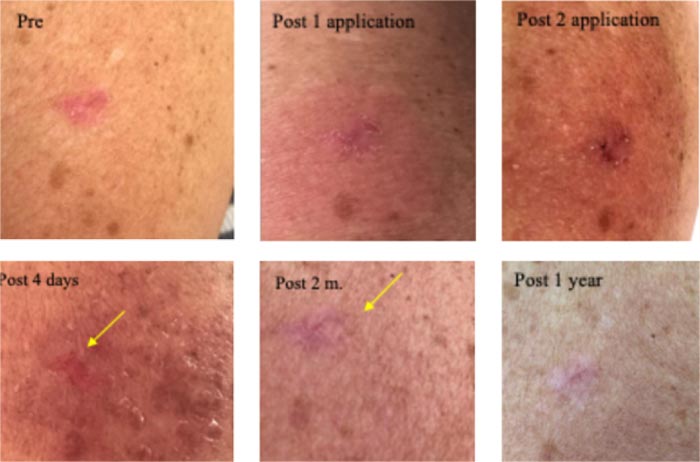
Clinically diagnosed as a superficially invasive BCC. The first 12-hour CPEEB application was a little irritating. The following application had to be discontinued after 6 hours (due to urgent travel). A picture was sent showing maceration of the lesion and irritation of surrounding sun-damaged skin. Another picture sent 4 days later showed partial recovery of irritation (after application of a mild steroid ointment) and lesion destruction. Pictures sent 2 months and one year later show normal skin with no apparent lesion and minimal scarring (Fig. 7).
4. DISCUSSION
In this paper, we report our preliminary experience of an off-label treatment of 3 patients with BCCs with a topical bromelain enriched concentrate of proteolytic enzymes. The Active Pharmaceutical Ingredient (API) that we used is approved as a safe and effective debriding agent for the treatment of deep burns (NexoBrid), and is in clinical trials of chronic wounds (EscharEx). As the patients could not be treated at the hospital due to the COVID-19 lockdown, this home-based telemedicine monitored, off-label treatment with an approved safe API seemed to be a valid alternative. We found that all six tumors were completely destroyed by the topical CPEEB, generally within 5-6 home applications over a period of up to 2 weeks. In one tumor, only two applications were required, with each application extending between 6-12 hours. Despite temporary mild-moderate local irritation and itching pain, the treatment was well-tolerated, and no analgesia was required. Our results emphasize the importance of individualizing treatment based on patient and tumor characteristics and response monitored by a skin specialist. All treated sites healed without hypertrophic scarring within 2-4 weeks. None of the treated tumors recurred 6-12 months after the treatment. The sites of two lesions underwent MOHS surgery 6 months after initial treatment, with no evidence of any residual tumor cells.
Our preliminary findings suggest that the treatment appears to be not only safe and effective but also feasible when administered by the patient or caregiver, with close supervision by a specialist with expertise treating skin cancers. Monitoring of the treatment was conducted both in person and by telemedicine, further supporting its potential use, especially in patients that have difficulty returning to the clinic for frequent reevaluations.
We believe that the ability of the CPEEB to destroy the BCC is in part due to the friable nature of the tumor, especially when compared with the surrounding denser and more firm skin. It is the potent proteolytic activity of the agent which is the basis for its ability to rapidly yet selectively debride deep burns after a single 4-hour application. This led us to hypothesize that CPEEB would similarly be able to “dissolve” the tumor by selectively destroying the bulk of the lesion without damaging the surrounding normal skin.
We did not expect the CPEEB molecular activity to affect all the tumor cells selectively via molecular pathways. However, together with its proteolytic activity that affects the tumor tissue matrix and bulk, we believed that it might destroy every single tumor cell, especially at the edges of the tumor, at the interface between the tumor and the normal skin. This is why we treated the tumor together with a small border of normal tissue (as in ‘surgical margins’) with the hope that the anti-tumor and pro-apoptotic effects of CPEEB might extend to any surrounding stray tumor cells, further reducing the risk of recurrence. We also hypothesized that the anti-inflammatory properties of CPEEB would contribute to the normal healing of the ulcerated wound bed after tumor destruction resulting in minimal scarring.
We recognize that our findings of the off-label treatment by CPEEB are limited by the small number of patients and tumors. As such, our findings should be interpreted as a preliminary proof-of-concept report that a concentrate of proteolytic enzymes enriched in bromelain, such as the API used in NexoBrid and EscharEx, may be a safe and effective treatment for cutaneous basal cell carcinomas. Additionally, the lesions in this preliminary report were diagnosed visually and by Dermatoscopy and were not confirmed histopathologically. Obviously, future well-designed extensive studies of this approach are necessary. We suggest that future studies of CPEEB be accompanied by pre and post-treatment tissue biopsies examining specimens for pre-treatment diagnosis and any signs of residual tumor cells after treatment.
CONCLUSION
Our preliminary findings provide initial proof-of-concept that treatment of BCCs with a concentrate of proteolytic enzymes enriched in bromelain may be a safe and effective treatment. Future studies are needed before any final conclusion is drawn.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The author confirms that the treatment in the study is an off label treatment of an approved PI (Principle Ingredient). Thus, it doesn't require approval of the ethical committee.
HUMAN AND ANIMAL RIGHTS
No Animals were used in this research. All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
Informed consent was taken from all the participants when they were enrolled.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest financial or otherwise.
ACKNOWLEDGEMENTS
We would like to thank Dr. Dafna Geblinger and Dr. Eilon Asculai for their contribution to this manuscript.


