All published articles of this journal are available on ScienceDirect.
Wound Healing Effects of Liposomal Nanocurcumin and PL Pro Nanocurcumin on Thermal Burn and Skin Ulcer
Abstract
Background
Burn injuries and skin ulcers are important health problems resulting in physical and psychological scars and chronic disabilities. This study investigated the wound-healing effects of liposomal nanocurcumin and PL pro nanocurcumin on thermal burns in rats and doxorubicin-induced skin ulcers in mice and their systemic toxicity.
Methods
Having subjected to a cylindrical hot steel rod onto the dorsum, burned lesions were covered topically with silver sulfadiazine/liposomal nanocurcumin/PL pro nanocurcumin twice a day for 21 days. Besides, the other skin lesions which were induced by a single intradermal injection of doxorubicin on the dorsal region were topically administered with dimethyl sulfoxide/liposomal nanocurcumin/PL pro nanocurcumin twice a day for 21 days.
Results
The results indicated that liposomal nanocurcumin and PL pro nanocurcumin significantly reduced the wound size, increased the hydroxyproline content in animals’ skin, and improved the histopathological structure of the affected tissues. Specifically, liposomal nanocurcumin demonstrated better healing results than PL pro nanocurcumin on thermal burns. Furthermore, topical administration of liposomal and PL pro nanocurcumin was deemed not to exert any systemic toxicity to the wounded animals by not influencing considerably the hematological parameters and renal and hepatic functions and altering the histology of the liver and kidney. Additionally, liposomal nanocurcumin and PL pro nanocurcumin with average sizes of 206 nm and 344 nm were well-dispersed in water, accentuating that the disadvantages of limited water solubility have been overcome.
Conclusion
Thus, liposomal nanocurcumin and PL pro nanocurcumin exerted effective effects on burned wounds and skin ulcers whilst triggering no systemic toxicity in wounded animals.
1. INTRODUCTION
Curcumin has perennially been referred to as a bioactive, important compound found in nature. To be specific, this substance is isolated from Curcuma longa L., which belongs to the Zingiberaceae species with a scientific name of (1e,6e)-1,7-bis(4-hydroxy-3’-methoxy phenyl)-1,6-heptadiene-3,5-dione [1, 2]. For years, curcumin has been profoundly embedded in the socio-cultural lifestyle of different peoples, especially Asians. It is not only considered a nature-based colorant, flavouring agent, and food preservative in many local cuisines but is also utilized for the sake of curing many illnesses. Regarding the pharmacological properties, many beneficial biological features of curcumin have been identified, such as anti-oxidant, anti-inflammatory, anti- microbial, anti-mutagenic, anti-tumoral, anti-angio- genesis activities, and wound healing effects [3-10]. Besides the evidence for the diversity of bioactivities of curcumin, this substance barely exhibits any toxicity at high doses when used for clinical treatment purposes [11-16]. Therefore, this statement does pave the way for curcumin to be popularly studied in various research studies relating to the dysregulation of many human organs. In particular, when it comes to some skin disorders and damages with many free radicals emitted, curcumin improves the skin with its capability to eradicate reactive oxygen species and attenuates local inflammation by inhibiting the nuclear receptor NF-κB [17]. Furthermore, treating skin disorders with curcumin does help to shorten the wound healing time, enhance the deposition of collagen, and also increase the density of fibroblasts and vasculatures, thus reinforcing the healing of the affected tissue with different levels of severity [18-20].
Notwithstanding those mentioned beneficial features, curcumin does exhibit many limitations, including poor water solubility and physicochemical instability, less bioactive absorption, rapid metabolization, and low penetration and targeting efficacy [21-24]. Meanwhile, nanoformulation has been testified regarding the potential of targeted delivery to the tissue of interest that leads to enhanced bioavailability and bioactivity and better drug carriage [25-28]. With the intention of taking advantage of this compound and simultaneously solving its drawbacks, the advent of nanocurcumin has shown to be prominent. There are currently several methods to encapsulate curcumin molecules at the nanoscale, and each uses a different but suitable nanocarrier [29].
Burn injuries and skin ulcers are still considered important health problems affecting both genders and all age groups, resulting in physical and psychological scars and leading to chronic disabilities [30-33]. To date, research on burns has generated sustained interest over the past few decades. In current burn therapy, silver sulfadiazine has been presented as the gold standard in topical second-degree burn treatment because of its antibacterial activities [34, 35]. However, the effect of silver sulfadiazine stems from the toxicity towards keratinocytes and fibroblasts, hence decelerating the wound healing process and probably triggering serious cytotoxic effects on the host cells. Furthermore, there are quite a number of articles to be reviewed on some emerging sliver-sulfadiazine-resistant organisms [36-38]. Additionally, in the treatment of skin ulceration, dimethyl sulfoxide (DMSO) is perennially one of the most proposed remedies since it can easily infiltrate into the affected area and scavenge free radicals, which is an important etiology of serious tissue damage [39]. However, for the time being, the accessibility of DMSO and other effective agents for skin ulcers is still restricted. Therefore, seeking a safer and more effective treatment approach towards skin lesions has been critically demanded in healthcare practice, particularly those caused by thermal or chemical triggers.
The beneficial effects and potentials of curcumin in different nano-based dosage forms, including liposomal nanocurcumin and PL pro nanocurcumin, with the assessment in terms of healing effects in both burned and ulcerated skin lesions, as well as the systemic toxicity in the experimental model remain unclear. In the present study, we investigated the wound-healing effect of liposomal nanocurcumin and PL pro nanocurcumin on thermal burns in rats and doxorubicin-induced skin ulcers in mice and their systemic toxicity in ulcerated experimental animals.
2. MATERIALS AND METHODS
2.1. Preparation of Liposomal Nanocurcumin and PL Pro Nanocurcumin Formula
2.1.1. Liposomal Nanocurcumin
Firstly, nanocurcumin was created. The dispersed phase by dissolving curcumin in ethanol was prepared with a volume ratio of 4/5. Then, a carrier mixture consisting of polyethylene glycol (PEG) and ethylene glycol by dispersing polyethylene glycol and ethylene glycol well in water was made with a ratio of approximately 1.5/6/2 for polyethylene glycol/ethylene glycol /water, under ultrasonic vibration for about 2 hours at room temperature. A homogeneous mixture was made by mixing the dispersed phase/liquid in the previous step, the carrier mixture, and the emulsifier lecithin such that the ratio of curcumin/PEG/lecithin in this homogenizer was 1.6/1.5/2 using an emulsifier. Nano-emulsions of curcumin were created by allowing the mixture to homogenize overnight and then centrifugated at room temperature at 5000 rpm for about 10 minutes, which was repeated six times. After obtaining curcumin nano-emulsions, nano-curcumin and phospholipids were weighed and prepared according to the respective ratio of 1/1. Liposomal nanocurcumin was obtained by putting the prepared mixture into the emulsifier and heating it at 120oC within 4 hours.
2.1.2. PL Pro Nanocurcumin
Nano curcumin was prepared using the method described above. PL pro included 18% phosphatidyl- choline, 21% cholesterol, 27% lecithin, 9.5% folic acid, 15% nano curcumin, 3% tocopherol, 3% xanthan gum, 3% Camellia sinensis extract, and 0.5% Aloe vera extract, then nano curcumin and PL pro were mixed according to the corresponding volume ratio of 1/1 in the emulsifier. After two hours, PL pro nanocurcumin was obtained.
2.2. Particle Size
Liposomal nanocurcumin and PL pro nanocurcumin samples were tested to determine particle size. This process was carried out utilising Malvern Mastersizer (Malvern Instruments Ltd., United Kingdom). The measurement was carried out by dissolving particles in water before measuring. The system temperature was kept at about 25°C. Hence, the solution was checked in terms of limitations regarding solubility.
The stability of liposomal nanocurcumin and PL pro nanocurcumin was determined through an accelerated aging test. This test simulates the aging process over time by subjecting the samples to high temperatures to artificially expedite the aging process. The accelerated aging process was conducted using an incubator (Daihan Scientific, South Korea), maintaining a constant temperature of 40°C for six months.
2.3. Experimental Animals
Male and female Wistar rats weighing 180 ± 20g and seven-week-old male and female Swiss albino mice were obtained from the National Institute of Hygiene and Epidemiology, Hanoi, Vietnam. All experimental protocols were in accordance with the National Guideline (reference number: 141/QD-K2DT). This study was approved by the Scientific Board Committee of Hanoi Medical University, Vietnam (ref number: IRB00003121). All animals were housed in a controlled environment (25 ± 1ºC under 65 ± 5% humidity and a 12-hour light and dark cycle) with ad libitum to access the standard rodent diet and water. The animals were given for at least one week to acclimate before starting the experiments.
2.4. Healing Effect of Topical Administration of Liposomal Nanocurcumin and PL Pro Nanocurcumin Creams
2.4.1. Thermal Burn in Rats
We followed the previously reported model of thermal burns on rats. A total of 50 rats were randomly divided into five groups of ten animals. The rats were anesthetized with a single intraperitoneal injection of 250 mg/kg chloralhydrate (Sigma Aldrich, St. Louis, MO, USA). As preparation, they were shaven at the dorsum with an electric shaver and later sterilized with 70% alcohol. All animals, except the normal control group, were subjected to thermal burns on the back of each rat by using a standard burning technique [40]. Burn wounds were formed by applying a 200-gram cylindrical stainless-steel rod (2.5 cm diameter) without any pressure, which was pre-heated to 100°C in boiling water with the thermal equilibrium confirmed by a monitoring thermometer, onto the shaven skin for 35 seconds. All animals were resuscitated immediately with Lactated Ringer’s solution (2 ml/100 g body weight) intraperitoneally. Following the burning, each animal was placed in a separate cage, and the affected areas were covered with 0.3 g silver sulfadiazine, liposomal nanocurcumin, or PL pro nanocurcumin twice a day for 21 days. The vehicle-treated burned rats topically received sterile distilled water (Fig. 1A).
2.4.2. Doxorubicin-induced Skin Ulcer in Mice
Fifty mice were randomly divided into five groups of ten animals. Mice were anesthetized with an intraperitoneal injection of 350 mg/kg chloralhydrate. After anesthesia, the dorsal regions were shaven with an electric shaver and sterilized with 70% alcohol. All animals, except the normal control group, were induced skin ulcers by a single intradermal injection of 0.2 ml doxorubicin 1 mg/0.5 ml (Doxorubicin Ebewe, Austria) [41]. Then, each animal was placed in a separate cage. Seven days after the injection of doxorubicin, the vehicle-treated ulcerated mice topically received sterile distilled water. The other ulcerated mice were topically applied 0.3 ml DMSO (Sigma Aldrich, St. Louis, MO, USA) twice a day, 0.3 g liposomal nanocurcumin or PL pro nanocurcumin twice a day for 21 days (Fig. 1B).
2.4.3. Measurement of the Wound Size
Wound sizes of animals in two experiments were measured using a digital camera with one camera lens and from a constant focal distance. The area of the wound was measured in a blind manner using ImageJ basics software ver 1.38, which was recognized as software for measuring the area in medical experimental research by the World Health Organization.
2.4.4. Determination of the Hydroxyproline Content
At the end of two experiments, mice and rats were anesthetized with chloralhydrate, and skin samples were collected from each animal. The concentration of hydroxyproline in the skin was evaluated according to the Stegemann H. and Stalder K method [42]. Briefly, 20 to 30 mg of skin tissues were put into hydrolytic tubes with 2 mL HCl 6N. These tubes were incubated at 115°C. After 24 hours, the hydrolyzed fluid was collected into the test tubes. Each test tube included 0.2 mL hydrolyzed fluid of samples, 1.8 mL distilled water, and 1 mL chloramine T. These test tubes were shaken and kept at room temperature for 20 minutes. Then, 2 mL pechloric acid 4M was added, shaken well, and let stand for 5 minutes at room temperature. 4-Dimethylaminobenzaldehyde 10% was added, shaken well, and kept in a bain-marie at 60oC for 15 minutes. These tubes were cooled down to room temperature and measured the light of 560 nm wavelength absorption (Shimadzu, Japan).
2.4.5. Histopathological Evaluation
The ulcerated skin tissue samples were collected for histopathological examinations. Histopathological evaluation was carried out randomly in 30% of each group. These tissue samples were fixed in 10% neutral-buffered formalin solution before they were embedded in paraffin wax and cut into 5 μm-thick sections to be stained with hematoxylin and eosin (H&E). The pathologist who examined the slides was blind to group allocation. Under histopathological examinations, inflammation, epithelization, neovascularization, and necrosis were evaluated.
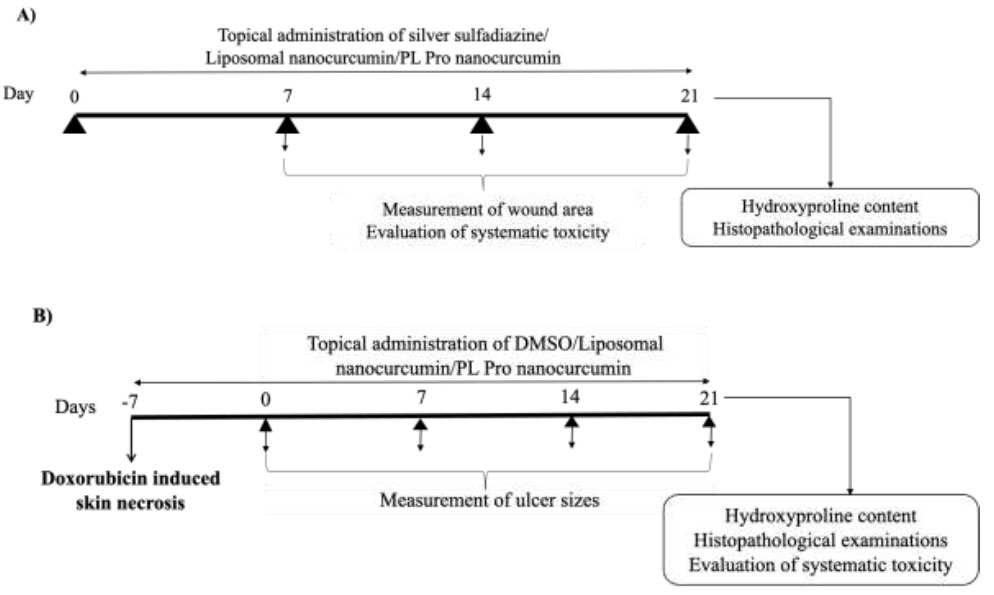
Experimental protocols. (A) Experimental protocol for evaluating the effects of topical administration of liposomal nanocurcumin and PL pro nanocurcumin creams on thermal burns in rats. (B) Experimental protocol for evaluating the effects of topical administration of liposomal nanocurcumin and PL pro nanocurcumin creams on doxorubicin-induced skin necrosis in mice.
2.5. Evaluation of systemic toxicity of topical administration of liposomal nanocurcumin and PL pro nanocurcumin creams in wounded animals
Blood samples were collected from each animal. The systemic effects were quantified through general conditions, including body weight changes in mice. Moreover, the hematopoietic function was evaluated through red blood cell count, hemoglobin, hematocrit, total white blood cells, and platelet count. The liver damage was examined through aspartate aminotrans- ferase level (AST) and alanine aminotransferase level (ALT), and the liver function was measured through total bilirubin, albumin, and total cholesterol. Furthermore, kidney function was examined through creatinine level. Follow-up parameters were checked at the time points before applying the products after 10 and 21 days in the thermal burn model in rats (Fig. 1A). In the doxorubicin-induced skin ulcer model in mice, blood samples were obtained after 21 days of treatment (Fig. 1B).
At the end of the experiments, animals were euthanized after blood collection, and the internal organs (heart, liver, spleen, kidney, and lung) were removed and observed for any gross lesions. The liver and kidneys of 30% of the animals in each group were preserved in a 10% buffered formaldehyde solution for histopathological studies using hematoxylin and eosin (H&E) staining by a researcher blinded to the study.
2.6. Data Analysis
Sigmaplot 12.0 (SYSTA Software Inc, Richmond, CA, USA) was used for statistical analysis. Obtained data were expressed as the mean ± S.D and compared with either one-way-ANOVA, followed by the post hoc Student-Newman-Keuls test for multiple comparisons or Fisher's Exact test for two proportions. Statistically significant differences were considered when the p-value was less than 0.05.
3. RESULTS
3.1. Particle Size
Liposomal nanocurcumin and PL pro nanocurcumin with average sizes of 206 and 344 nm were well-dispersed in water, indicating that the disadvantages of limited water solubility have been overcome. Furthermore, the results of the accelerated aging study revealed that after six months of accelerated aging at 40°C, both liposomal nanocurcumin and PL pro nanocurcumin remained stable in particle size (Fig. S1).
3.2. Healing Effects of Liposomal Nanocurcumin and PL Pro Nanocurcumin on Thermal Burns in Rats
3.2.1. Effect on the Wounded Area
As shown in Fig. (2), after 7 days of treatment, there was no difference in burned area between groups. After 14 days of administration, silver sulfadiazine, liposomal nanocurcumin, and PL pro nanocurcumin markedly reduced the wounded area compared to the vehicle-treated model group (vehicle-treated burned group vs. silver sulfadiazine-treated burned group, p=0.003; vehicle-treated burned group vs. liposomal nanocurcumin-treated group, p<0.001; vehicle-treated burned group vs. PL pro nanocurcumin-treated group, p=0.003). The burned area of liposomal nanocurcumin-treated rats significantly decreased compared with the PL pro nanocurcumin-treated rats (p=0.006).
After 21 days of treatment, the burn lesions of vehicle-treated rats were not completely healed. The rate of burn wound healing in the silver sulfadiazine- and liposomal nanocurcumin-treated groups was 50%, with a statistically significant difference compared to the vehicle-treated group (p=0,033; Fisher’s exact test). PL pro nano- curcumin-treated rats had a wound healing rate of 20%. There was no markedly significant difference in the rate of wound healing between the vehicle-treated group and the PL pro nanocurcumin-treated group (p>0.05).
3.2.2. Effect on Hydroxyproline Content
As shown in Fig. (3), the content of hydroxyproline in the rat skin sample of the vehicle-treated group was significantly lower than the normal control group (p<0.001). Compared with the vehicle-treated model group, treatment of silver sulfadiazine, liposomal nanocurcumin, and PL pro nanocurcumin was found to increase the level of hydroxyproline in the skin tissue (vehicle-treated burned group vs. silver sulfadiazine-treated burned group, p=0.002; vehicle-treated burned group vs. liposomal nanocurcumin-treated group, p<0.001; vehicle-treated burned group vs. PL pro nanocurcumin-treated group, p<0.001).
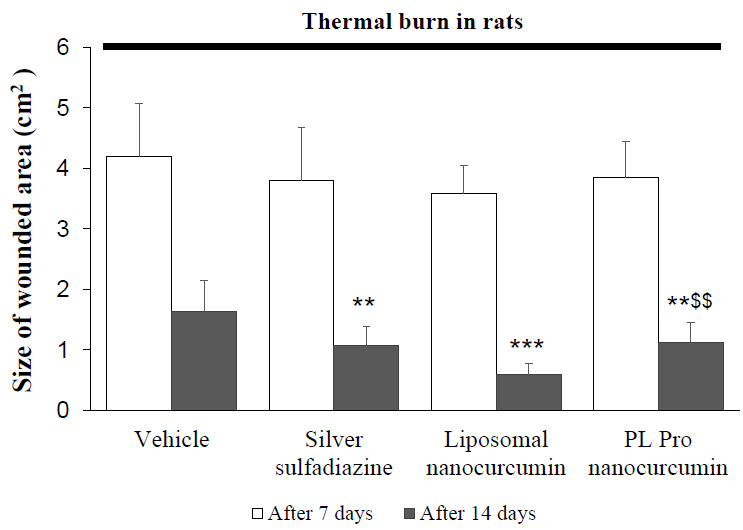
Effects of liposomal nanocurcumin and PL pro nanocurcumin on the size of the wounded area in burned rats. Data were expressed as the mean ± S.D. (n=10). *p<0.05, **p<0.01, ***p<0.001: compared to vehicle-treated burned rats. $p<0.05, $$p<0.01, $$$p<0.001: compared to liposomal nanocurcumin-treated burned rats (One-way-ANOVA followed by post hoc Student-Newman-Keuls test)
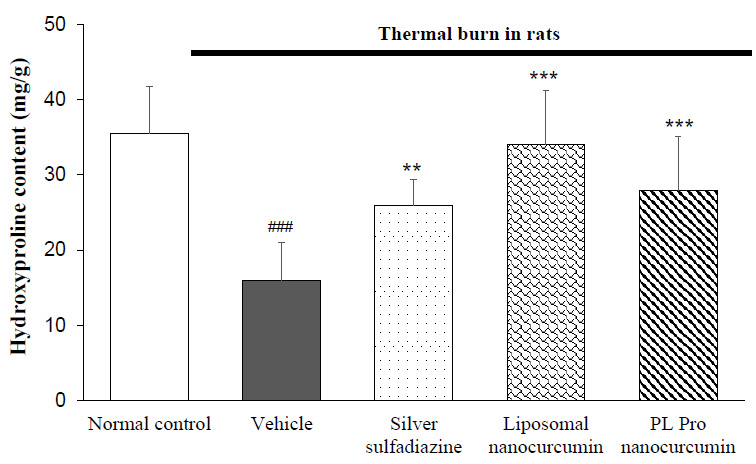
Effects of liposomal nanocurcumin and PL pro nanocurcumin on hydroxyproline content in skin tissues in burned rats. Data were expressed as the mean ± S.D. (n=8). *p<0.05, **p<0.01, ***p<0.001: compared to vehicle-treated burned rats. #p<0.05, ##p<0.01, ###p<0.001: compared to normal control group (One-way-ANOVA followed by post hoc Student-Newman-Keuls test)
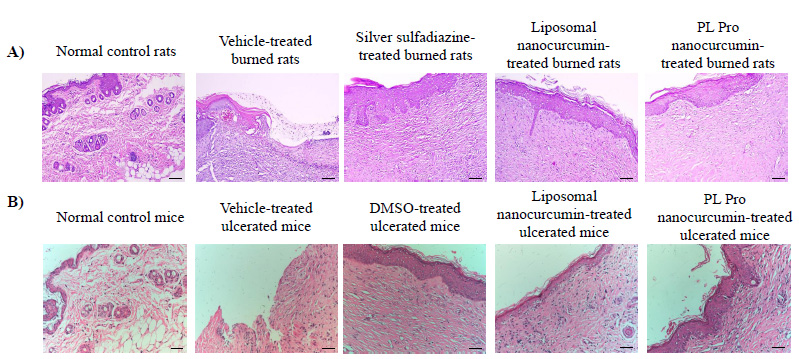
(A) The microscopic representative images of burned wounds in rats. (B) The microscopic representative images of skin ulcers in mice. Black bars in each photo indicate 100 μm.
3.2.3. Histopathological Examination
The skin biopsy of the normal control rats demonstrated the proper and well-structured stratum epidermis with keratinization, clear basal lamina, skin-dependent components in the dermis layer, loose connective tissue, and small blood vessels. Thus, in the normal control group, the skin structure of rats was found to be normal. In the vehicle-treated group, the rat skin tissue showed a large ulcerated area whilst the surface was covered with the necrotic substance erythrocyte fibrin, various inflammatory cells, neutrophils, and macrophages. On the 21st day, burn healing was better in silver sulfadiazine, liposomal nanocurcumin, and PL pro nanocurcumin-treated groups than in the vehicle-treated group (Fig. 4A-B).
3.3. Evaluation of Systemic Toxicity of Topical Administration of Liposomal Nanocurcumin and PL Pro Nanocurcumin Creams in Burned Rats
During the experimental period, there was an increase in body weight in each group of animals. No significant differences were found as compared to that of the control group. None of the animals in all treated groups showed any macroscopic or gross pathological changes when compared to the control group. No gross lesions or changes in size were observed when evaluating all experimental rats to a full gross necropsy, which examined the hearts, livers, lungs, kidneys, and abdominal cavities.
3.3.1. Effect on Hematopoietic Function
As mentioned in Table 1, there were no significant differences in red blood cell count, hematocrit, hemoglobin level, platelet count, and total white blood cells between liposomal nanocurcumin and PL pro nanocurcumin-treated groups and normal control group (p>0.05).
3.3.2. Effect on Liver Damage, Liver Function, and Kidney Function
Fig. (5A1-2 and 5B) demonstrate that liposomal nanocurcumin and PL pro nanocurcumin did not cause any statistical difference in AST, ALT levels, and creatinine levels when comparing the treated groups to the normal control group (p>0.05). The effect of liposomal nanocurcumin and PL pro nanocurcumin on the total bilirubin, albumin, and total cholesterol of the normal control group and treated groups are presented in Table 2. No statistical difference was observed between groups (p>0.05). In addition, there were no significant differences in histopathological examinations of livers and kidneys between liposomal nanocurcumin and PL pro nanocurcumin-treated rats and the normal control group (Fig. S2A).
3.4. Healing Effects of Liposomal Nanocurcumin and PL Pro Nanocurcumin on Doxorubicin-induced Skin Ulcer in Mice
3.4.1. Effect on Ulcerated Area
As shown in Fig. (6), no difference in the areas of skin ulcers was found between groups (p>0.05) for the time before treatment. After 7 and 21 days of administration, DMSO, liposomal nanocurcumin, and PL pro nanocurcumin significantly reduced the ulcer size compared to the vehicle-treated group (p<0.01). Additionally, there were no statistical differences in terms of reducing the skin lesions’ area between liposomal nanocurcumin and PL pro nanocurcumin (p>0.05).
| Parameters | Groups | n | Before Treatment |
After 10 Days |
After 21 Days |
|---|---|---|---|---|---|
| Rbc (T/L) | Normal control | 10 | 8.74 ± 0.50 | 8.63 ± 0.98 | 9.29 ± 0.67 |
| Liposomal nanocurcumin | 10 | 9.03 ± 0.50 | 8.37 ± 1.09 | 9.56 ± 0.71 | |
| PL Pro nanocurcumin | 10 | 8.29 ± 0.98 | 8.76 ± 1.17 | 9.14 ± 1.24 | |
| Hemoglobin (G/dl) | Normal control | 10 | 11.14 ± 0.79 | 10.87 ± 0.55 | 11.75 ± 0.66 |
| Liposomal nanocurcumin | 10 | 11.74 ± 0.88 | 10.94 ± 1.32 | 11.85 ± 1.01 | |
| PL Pro nanocurcumin | 10 | 11.02 ± 0.88 | 10.84 ± 1.05 | 11.56 ± 0.86 | |
| Hematocrit (%) | Normal control | 10 | 46.53 ± 3.11 | 45.21 ± 3.44 | 48.74 ± 2.99 |
| Liposomal nanocurcumin | 10 | 48.95 ± 3.19 | 47.51 ± 3.72 | 50.63 ± 3.80 | |
| PL Pro nanocurcumin | 10 | 48.32 ± 4.96 | 47.80 ± 3.38 | 50.21 ± 2.26 | |
| Mean corpuscular volume (fl) | Normal control | 10 | 53.00 ± 1.83 | 52.30 ± 1.64 | 52.40 ± 1.90 |
| Liposomal nanocurcumin | 10 | 52.50 ± 1.84 | 51.70 ± 2.21 | 52.80 ± 1.75 | |
| PL Pro nanocurcumin | 10 | 51.90 ± 1.37 | 50.60 ± 2.67 | 51.60 ± 1.84 | |
| White blood cell (G/L) | Normal control | 10 | 6.77 ± 1.34 | 7.26 ± 1.17 | 7.68 ± 0.87 |
| Liposomal nanocurcumin | 10 | 6.52 ± 1.96 | 6.85 ± 1.83 | 7.43 ± 1.64 | |
| PL Pro nanocurcumin | 10 | 6.67 ± 1.11 | 7.54 ± 1.53 | 7.71 ± 1.84 | |
| Platelets (G/L) | Normal control | 10 | 527.70 ± 92.24 | 546.70 ± 89.19 | 532.00 ± 88.15 |
| Liposomal nanocurcumin | 10 | 590.60 ± 97.91 | 510.90 ± 88.16 | 602.50 ± 93.89 | |
| PL Pro nanocurcumin | 10 | 509.40 ± 96.59 | 529.10 ± 101.56 | 515.10 ± 85.62 |
| - | Groups | n | Before Treatment |
After 10 Days |
After 21 Days |
|---|---|---|---|---|---|
| Total bilirubin (mmol/l) | Normal control | 10 | 9.14 ± 0.64 | 9.38 ± 0.75 | 9.27 ± 0.75 |
| Liposomal nanocurcumin | 10 | 9.63 ± 0.84 | 9.68 ± 0.92 | 9.54 ± 1.08 | |
| PL Pro nanocurcumin | 10 | 9.43 ± 0.78 | 9.17 ± 0.68 | 9.33 ± 0.42 | |
| Albumin (g/dl) | Normal control | 10 | 3.17 ± 0.46 | 3.22 ± 0.24 | 3.07 ± 0.27 |
| Liposomal nanocurcumin | 10 | 3.36 ± 0.28 | 3.15 ± 0.28 | 3.14 ± 0.29 | |
| PL Pro nanocurcumin | 10 | 3.07 ± 0.26 | 3.19 ± 0.23 | 2.92 ± 0.29 | |
| Total cholesterol (mmol/l) | Normal control | 10 | 1.43 ± 0.25 | 1.38 ± 0.10 | 1.49 ± 0.21 |
| Liposomal nanocurcumin | 10 | 1.57 ± 0.22 | 1.45 ± 0.15 | 1.60 ± 0.15 | |
| PL Pro nanocurcumin | 10 | 1.35 ± 0.12 | 1.40 ± 0.18 | 1.46 ± 0.19 |
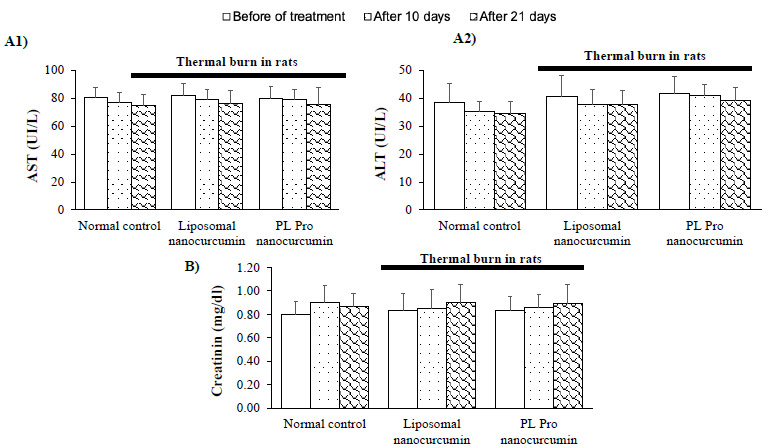
Effect of liposomal nanocurcumin and PL pro nanocurcumin on liver damage (A1-2) and kidney function (B) in burned rats. Data were expressed as the mean ± S.D. (n=10). #p<0.05, ##p<0.01, ###p<0.001: compared to normal control group (One-way-ANOVA followed by post hoc Student-Newman-Keuls test)
After 21 days of administration, the rate of wound healing in vehicle-treated mice was 10%. The rate of wound healing in the silver sulfadiazine- and the liposomal nanocurcumin-treated group was 70% and 80%, respectively (p=0.02 and p=0.005 compared to the vehicle-treated group; Fisher’s Exact test). PL pro nanocurcumin-treated mice had a wound healing rate of 60%. There was no noticeably significant difference in the rate of wound healing between the vehicle-treated group and the PL pro nanocurcumin-treated group (p=0.057).
3.4.2. Effect on Hydroxyproline Content
The hydroxyproline content is presented in Fig. (7). The hydroxyproline level in skin tissues of the vehicle-treated group was significantly lower than the normal control group (p<0.001). Compared with the vehicle-treated model group, treatment of DMSO, liposomal nanocurcumin, and PL Pro nanocurcumin significantly increased the hydroxyproline content in the skin tissue. In addition, there were no significant differences in the effects of liposomal nanocurcumin and PL pro nanocurcumin on the concentration of hydroxyproline in skin tissues (p>0.05).
3.4.3. Histopathological Examination
The skin biopsy of the normal control mice was normal, with the proper stratum epidermis with keratinization, clear basal lamina, skin-dependent components in the dermis layer, loose connective tissue, and small blood vessels. In the vehicle-treated ulcerated mice, the skin tissue showed a large necrosis area, and the surface was covered with necrotic substances, erythrocytes, fibrin, many inflammatory cells, neutrophils, and macrophages. On the 21st day, DMSO, liposomal nanocurcumin, and PL pro nanocurcumin improved the histopathological structure of skin tissues, which demonstrated the slight growth of dermal papillae and epidermal ridges.
3.5. Evaluation of Systemic Toxicity of Topical Administration of Liposomal Nanocurcumin and PL Pro Nanocurcumin in Ulcerated Mice
During the experimental period, there was an increase in body weight in each group of animals. No significant differences were seen as compared to that of the control group. None of the animals in all treated groups showed any macroscopic or gross pathological changes when compared to the control group. No gross lesions or changes in size were observed when evaluating all experimental rats to a full gross necropsy, which examined the hearts, livers, lungs, kidneys, and abdominal cavities.
3.5.1. Effect on Hematopoietic Function
As mentioned in Table 3, there were no significant differences in red blood cell count, hematocrit, hemoglobin level, total white blood cell, and platelet count between liposomal nanocurcumin and PL pro nanocurcumin-treated groups and normal control group (p>0.05).
3.5.2. Effect on Liver Damage, Liver Function, and Kidney Function
Fig. (8) demonstrates that liposomal nanocurcumin and PL pro nanocurcumin did not cause any statistical difference in AST, ALT level, and creatinine levels when comparing the treated groups to the normal control group (p>0.05). The effects of liposomal nanocurcumin and PL pro nanocurcumin on the total bilirubin, albumin, and total cholesterol of the normal control group and treated groups are presented in Table 4. No statistical difference was observed between groups (p>0.05).
Additionally, there were no significant differences in histopathological examinations of livers and kidneys between liposomal nanocurcumin and PL pro nano- curcumin-treated ulcerated and normal control mice (Fig. S2B).
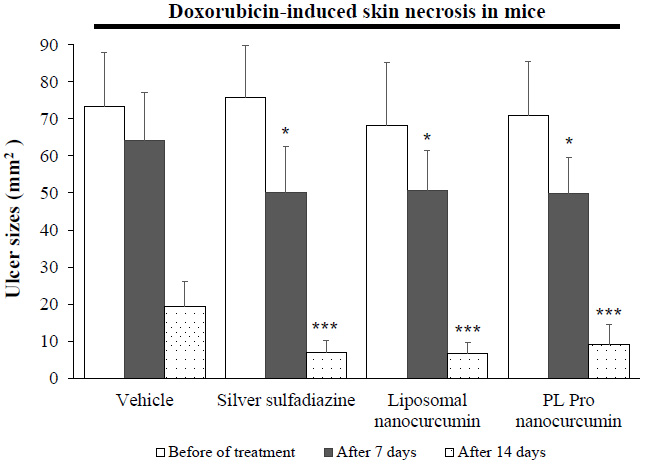
Effects of liposomal nanocurcumin and PL pro nanocurcumin on ulcer sizes in ulcerated mice. Data were expressed as the mean ± S.D. (n=10). *p<0.05, **p<0.01, ***p<0.001: compared to vehicle-treated ulcerated mice. $p<0.05, $$p<0.01, $$$p<0.001: compared to liposomal nanocurcumin-treated ulcerated mice (One-way-ANOVA followed by post hoc Student-Newman-Keuls test)
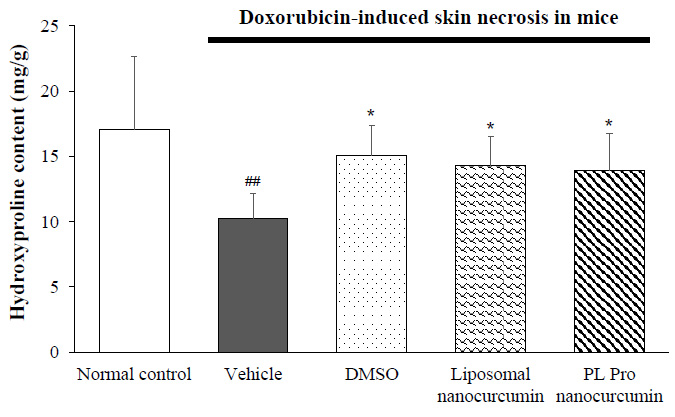
Effects of liposomal nanocurcumin and PL pro nanocurcumin on hydroxyproline content of skin tissues in ulcerated mice. Data were expressed as the mean ± S.D. (n=8). *p<0.05, **p<0.01, ***p<0.001: compared to vehicle-treated ulcerated mice. #p<0.05, ##p<0.01, ###p<0.001: compared to normal control group (One-way-ANOVA followed by post hoc Student-Newman-Keuls test)
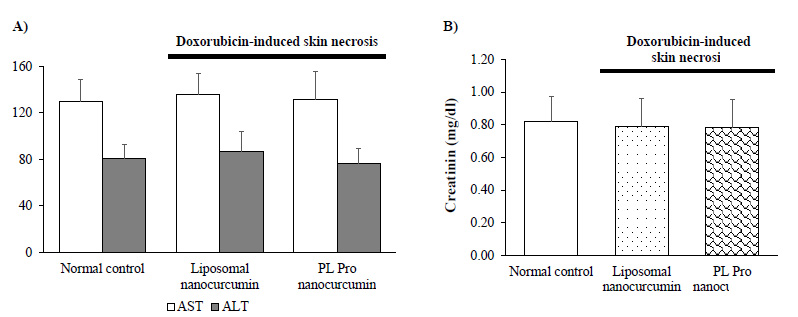
Effect of liposomal nanocurcumin and PL pro nanocurcumin on liver damage (A) and kidney function (B) in ulcerated mice. Data were expressed as the mean ± S.D. (n=10). #p<0.05, ##p<0.01, ###p<0.001: compared to normal control group (One-way-ANOVA followed by post hoc Student-Newman-Keuls test).
4. DISCUSSION
In this study, we not only evaluated the effect of topical administration of liposomal nanocurcumin and PL pro nanocurcumin on two different models of skin lesions, which were induced, respectively, by heat and doxorubicin but also detected any systemic toxicity of liposomal and PL pro nanocurcumin via the ulcers in the experimental animals. Our results showed that liposomal nanocurcumin and PL pro nanocurcumin significantly reduced the size of the wounded area, increased the hydroxyproline level in skin tissues, and improved the histopathological structure of skin tissues. Liposomal nanocurcumin showed better effects than PL pro nanocurcumin on thermal burns in rats. Additionally, topical administration of liposomal nanocurcumin and PL pro nanocurcumin did not cause systemic toxicity. Thus, liposomal nanocurcumin and PL pro nanocurcumin have been shown to accelerate the wound healing process without systemic toxicity in experimental rat models.
| Groups |
Normal Control (n=10) |
Liposomal Nanocurcumin (n=10) |
PL Pro Nanocurcumin (n=10) |
|---|---|---|---|
| Rbc (T/L) | 8.95 ± 1.55 | 8.74 ± 1.28 | 8.43 ± 1.08 |
| Hemoglobin (g/dl) | 9.50 ± 1.66 | 9.09 ± 1.43 | 9.54 ± 1.15 |
| Hematocrit (%) | 43.05 ± 5.35 | 40.31 ± 5.13 | 41.67 ± 4.70 |
| Mean corpuscular volume (fl) | 46.40 ± 2.32 | 46.00 ± 2.21 | 48.10 ± 2.18 |
| White blood cell (G/L) | 6.07 ± 1.58 | 5.83 ± 1.05 | 5.04 ± 1.31 |
| Platelets (G/L) | 821.20 ± 88.15 | 861.70 ± 92.80 | 850.10 ± 80.55 |
| - |
Normal Control (n=10) |
Liposomal Nanocurcumin (n=10) |
PL Pro Nanocurcumin (n=10) |
|---|---|---|---|
| Total bilirubin (mmol/l) | 9.65 ± 0.65 | 10.17 ± 0.92 | 9.85 ± 0.82 |
| Albumin (g/dl) | 2.95 ± 0.35 | 3.23 ± 0.33 | 3.14 ± 0.30 |
| Total cholesterol (mmol/l) | 2.27 ± 0.34 | 3.14 ± 0.30 | 2.02 ± 0.19 |
Thermal burn injuries and skin ulcers are still considered major health problems, resulting in physical and psychological scars and disabilities [30]. Depending on the lesion severity, wound healing is one of the most complex processes, which involves several phases of coagulation, inflammation, growth, re-epithelialization, and remodeling [43, 44]. Research on burns has generated sustained interest over the past few decades. However, drugs for treating burns and skin ulcers are still limited [36]. For the treatment of burns, silver sulfadiazine is considered the gold standard in the topical treatment of second-degree burns because of its antibacterial properties. However, silver sulfadiazine is associated with toxicity to keratinocytes and fibroblasts. So, this drug delays the wound healing process and has some serious cytotoxic effects on the host cells. Moreover, several bacteria are resistant to silver sulfadiazine [36]. Additionally, for the treatment of skin ulcers, dimethyl sulfoxide (DMSO) is a perennially proposed remedy since it can easily infiltrate into the affected area and scavenge free radicals [39]. However, currently, the pharmaceutical form of DMSO is limited. Moreover, the accessibility of DMSO in particular and other effective agents for skin ulcers is still restricted. Considering this, there is an emerging demand for a safer and more effective approach to be applied in the treatment of wounds.
Curcumin possesses a powerful wound-healing effect for the treatment of various skin disorders and damages [5, 45-47]. In particular, curcumin attenuates the inflammatory response and hastens wound healing by increasing cellular proliferation and improving collagen deposition in the wound tissues, as well as promoting angiogenesis in chronic wounds [48-50]. Thus, curcumin reinforces the healing of the affected tissue with different levels of severity. Effect on inflammation, fibroblast proliferation, granulation tissue formation, and collagen deposition are mentioned mechanisms of the healing potential of curcumin [51-53]. However, curcumin exhibits several limitations in wound healing treatment, including poor water solubility and physicochemical instability [21]. As a solution, nanoformulations should be applied in order to deliver substance to the targets more accurately [20]. Specifically, liposomes with nano-sized phospholipid bilayered vesicles were utilized for transport with a variety of drugs, including wound healing agents. These are not difficult to prepare and are highly biocompatible in nature. This approach of nanoformulation has shown promising results in the improvement of aqueous solubility of curcumin and the development of a sustained and prolonged drug-release system, thus enhancing wound healing and closure [54]. In the present study, thermal burn wounds in rats and doxorubicin-induced skin ulcers in mice were used to evaluate the healing effect of topical administration of liposomal nanocurcumin and PL pro nanocurcumin. Liposomal nanocurcumin and PL pro nanocurcumin significantly reduced the size of the wounded area, increased the hydroxyproline content in skin tissues, and improved the histopathological structure of skin tissues. In addition to assessing the criteria for the damaged area of skin ulcer, we also evaluated the hydroxyproline level. According to the literature, collagen plays a pivotal role in wound healing. Hydroxyproline is a major component of the protein collagen, as it is a principal component of connective tissues produced by fibroblasts. It assists the wound in gaining tensile strength during wound repair, hence serving as a structural framework, strength, and milieu for the regenerating tissue [55-68]. We determined collagen synthesis indirectly by hydroxyproline level. Our results indicated that liposomal nanocurcumin and PL pro nanocurcumin increased the level of hydroxyproline in the skin tissue.
In this study, doxorubicin, a chemotherapeutic drug belonging to the anthracyline group, was used as a skin ulcerative agent. It is one of the most important drugs causing skin necrosis and, ultimately, severe ulceration, with the incidence of extravasation injury being 0.1% to 6.5% [41, 59, 60]. This agent could affect the replication and translation process, as well as activate the gene that is responsible for cellular apoptosis. Eventually, the ulcer caused by doxorubicin injection was broad and deep, indicating that the ulceration model triggered by doxorubicin was adequately reliable in gauging the efficiency of liposomal nanocurcumin and PL pro nanocurcumin [61]. According to our previous study, the development of skin necrosis reached its maximum size in one week [62]. In order to alleviate the triggered skin lesion, the newly formed radicals in the cytosol and interstitial space should be eliminated by potent antioxidants for clinical practice. Therefore, DMSO was used as a positive control in this study. Our results indicated that liposomal nanocurcumin and PL pro nanocurcumin significantly reduced ulcer size. However, there were no significant differences in the healing effect between liposomal nanocurcumin and PL pro nanocurcumin-treated groups. In addition, burns can be defined as tissue lesions resulting from exposure to thermal sources, such as flames, hot surfaces and liquids, extreme cold, chemicals, radiation, or friction [32]. In this study, the model of superficial second-degree burns on rats was successfully induced. Interestingly, liposomal nanocurcumin showed better effects than PL pro nanocurcumin on thermal burns in rats. In addition, liposomal nanocurcumin significantly reduced infection, compared to the vehicle-treated group, by improving the macroscopic and histopathological structure. Further- more, it did not affect the number of white blood cells when compared to the normal control group. These effects underscore the efficacy of liposomal nanocurcumin in burn treatment. Thus, liposomal nanocurcumin is a more potent wound-healing agent.
We also evaluated the systemic toxicity after the application of topical liposomal nanocurcumin and PL pro nanocurcumin on thermal burns in rats and skin ulcers in mice. Long-term topical application can also affect the systemic effects, especially when applied to open wounds [63]. Overall, the findings of this study indicated that topical administration of liposomal nanocurcumin and PL pro nanocurcumin caused no significant change in the general status, haematological parameters, and renal and hepatic functions. Additionally, they did not alter the histology of the liver and kidneys in animals. In oral administration, curcumin did not exert acute, subchronic, chronic toxicity, or reproductive toxicity in animals [11, 12, 64]. To date, there have been no studies evaluating the systemic toxicity of nanocurcumin in open wounds. Our results indicated that liposomal nanocurcumin and PL pro nanocurcumin did not cause systemic toxicity in burned rats and ulcerated mice. Hence, these studies suggest the beneficial effects of liposomal nanocurcumin and PL pro nanocurcumin and the potential of these formulations to be developed as a potent nontoxic agents for treating skin disorders. Overall, liposomal nanocurcumin and PL pro nanocurcumin are valuable in the near future for wound healing, but additional studies are required to provide scientists with a deeper understanding.
CONCLUSION
The current study demonstrated that the topical application of liposomal nanocurcumin and PL pro nanocurcumin creams exerted healing effects on burned skin in rats and doxorubicin-induced skin ulcers in mice. Furthermore, liposomal nanocurcumin and PL pro nanocurcumin did not cause systemic toxicity in the experimental model. Liposomal nanocurcumin showed better effects than PL pro nanocurcumin on thermal burns in rats.
AUTHORS' CONTRIBUTIONS
It is hereby acknowledged that all authors have accepted responsibility for the manuscript's content and consented to itssubmission. They have meticulously reviewed all results and unanimously approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| DMSO | = Dimethyl Sulfoxide |
| AST | = Aspartate Aminotransferase |
| ALT | = Alanine Aminotransferase |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the Scientific Board Committee of Hanoi Medical University, Vietnam (ref number: IRB00003121).
HUMAN AND ANIMAL RIGHTS
All experimental protocols were in accordance with the National Guidelines (reference number: 141/QD-K2DT). This study adhered to internationally accepted standards for animal research, following the 3Rs principle. The ARRIVE guidelines were employed to report experiments involving live animals and promote ethical research practices.


