All published articles of this journal are available on ScienceDirect.
A Study on the Effectiveness and Safety of Herbal Extract Combination Compared to 3% Minoxidil Solution for the Treatment of Androgenetic Alopecia: A Randomized, Double-blind, Controlled Trial
Abstract
Background
Androgenetic alopecia (AGA) affects both men and women. Standard treatments include topical minoxidil and oral finasteride, which can cause undesirable side effects. Therefore, a natural herbal extract is required for an alternative treatment.
Objectives
This study aims to assess both the efficacy and safety profiles of an herbal extract comprising a combination of dihydroquercetin glucoside, epigallocatechin gallate glucoside, zinc, and glycine, compared to the minoxidil solution for the treatment of AGA.
Methods
About 30 males and 30 females were recruited. The males and females were divided equally into two groups (n=30), randomly receiving either a minoxidil solution or herbal extraction twice a day for 24 weeks. Clinical efficacy, total hair count, and hair mass index (HMI) were evaluated.
Results
A total of 30 males and 30 females completed the study. The total hair counts (hairs/cm2) at baseline in the herbal group and minoxidil group were 334.8±108.8 and 368.3±178.4, respectively, and the total hair counts at 24 weeks in the herbal group and minoxidil group were 345.0±119.2 and 391.5±183.1 (p<0.001, p<0.001), respectively. The HMI in the herbal and minoxidil groups significantly increased by 25.83±17.18 and 33.70±15.17 (p=0.001, p<0.001) at 24 weeks. However, there was no significant difference in total hair count and HMI between the two groups at 24 weeks (p= 0.250, p=0.065). No local adverse effects were observed in both groups.
Conclusions
The non-significant difference in efficacy and safety to minoxidil solution suggests that the herbal extraction could be an alternative treatment for AGA.
1. INTRODUCTION
Androgenetic alopecia (AGA) is one of the most common types of non-scarring alopecia, affecting up to 80% of men and 50% of women [1]. Dihydrotestosterone (DHT) on androgen-sensitive hair follicles causes the gradual miniaturization of hair follicles, resulting in an increased ratio of telogen hair, vellus transformation, and hair thinning. There are distinctive patterns of hair loss in men and women, classified accordingly by the Hamilton-Norwood pattern and Ludwig pattern. Diffuse thinning in the vertex and crown regions in men, and the parietal area in women is predominantly observed [2-5].
The current FDA-approved treatments for AGA in both sexes are: 2% minoxidil solutions in females and 5% minoxidil solution, including 1mg oral finasteride in males [3, 4]. Finasteride is a 5αR2 inhibitor [2] that suppresses type II 5-alpha reductase enzymes, thus reducing DHT levels and decreasing hair loss, whereas a topical minoxidil solution increases cutaneous blood flow, increases vascular endothelial growth factor (VEGF) levels, and enhances hair growth at the dermal papilla [3]. However, long-term oral finasteride can cause adverse side effects, including gynecomastia, sexual dysfunction, and reduced libido [2, 3], while the side effects of topical minoxidil solution include facial hypertrichosis and contact dermatitis. The latter is a consequence of propylene glycol, an ingredient in the vehicle [3].
Due to the unpleasant side effects experienced in these mainstay treatments, a safe and equally effective alternative treatment is worth investigating. Natural herbal extraction products that can enhance hair growth without adverse effects are desirable. The herbal extraction evaluated in this study was comprised of dihydroquercetin-glucoside or DHQG (from pine), epigallocatechin gallate glucoside or EGCG2 (from green tea leaves), glycine, and zinc. The ingredients in the herbal extract were sourced from Switzerland and Japan, and the quality was consistently maintained by standardized authorities. The ingredients were also approved by Good Manufacturing Practices (GMP) and currently hold documentation from a Certificate of Analysis (COA). The objective of the present study was to compare the efficiency and adverse effects of this natural product with the standard treatment for AGA.
2. MATERIALS AND METHODS
A total of 30 males (Norwood-Hamilton classification of IIa to V) and 30 females (Ludwig classification of I-4 to II-2), aged 18-65, were recruited for this study at Panyananthaphikku Chonprathan Medical Centre from July 2018 to February 2022; method of definition of sex is by self-report. Both groups of males and females were divided equally into two groups, which randomly received a 3% minoxidil solution (designated as the minoxidil group) or the herbal extraction (designated as the herbal group) (Redenyl®, Sino Pharm Co., Ltd., Bangkok Thailand) via a computer-generated block randomization method. The subjects and care providers were blinded. The subjects were instructed to apply 1 mL of topical medication twice daily for 24 weeks. The two medications were prepared in identical bottles. Both medications were clear and colourless solutions. The exclusion criteria were as follows: previous treatment with topical or systemic medications within six months, hair transplantation, current usage of any products or shampoo for hair growth, medical conditions (including autoimmune disease, thyroid disease, and immunocompromised host), and pregnancy/lactation. Standardized global photography and total hair counts were evaluated at baseline, 8 weeks, 16 weeks, and 24 weeks.
Standardized global photography taken from a digital camera (DSLR Canon EOS 80D, Japan) was evaluated by two blinded dermatologists using a standardized 7-point rating score (-3: significantly decreased, -2: moderately decreased, -1: slightly decreased, 0: no change, 1: slightly increased, 2: moderately increased, 3: significantly increased).
The total hair count was measured on a 1 cm2 area, in which a balding area on the mid-scalp was selected, hairs were trimmed, and the area was marked with a medical blue tattoo by an aseptic technique. Detailed photographs of the site were taken by dermoscopy (Dermlite III; 3 Gen LLC, CA, USA) on the tattooed area to evaluate the total hair count at each subsequent visit.
The HMI, representing hair density, was performed using a hair check system® (Divi Inter Co. Miami, FL, US) by measuring an area sized 2x2 cm2 at baseline and 24 weeks.
Demographic data were analysed using percentages and mean ±SD. Statistical analysis was performed with the SPSS software, version 16 (SPSS Inc., Chicago, IL, USA). Independent t-test, Fisher’s Exact test, and Chi-square were used for statistical evaluation. The p-value < 0.05 was considered as the cut-off for statistical significance.
3. RESULTS
Thirty men (mean aged 48.5±14.1) and thirty women (mean aged 54.8±12.4) completed the study (Fig. 1). Norwood-Hamilton classification II-a, III vertex, IV, IV-a, and V were 6.6%, 16.6%, 6.6%, 13.3%, and 56.6%, respectively. Ludwig classification I-4, II-1 and II-2 were 20%, 36.7% and 43.3%, respectively. The duration of onset in male and female subjects was 14.9 (6-28 years) and 11.5 (3-23 years), respectively. The subjects had no previous treatment with minoxidil solution or oral finasteride. There was no statistically significant baseline demographic data between the two groups (Table 1).
Table 1.
| Data Mean (SD) |
Herbal Group (n=30) |
Minoxidil Group (n=30) |
p-value | |
|---|---|---|---|---|
| Age, yrs | 51.7±13.564 | 50.73±12.498 | 52.67±14.702 | 0.585* |
| Male | ||||
| Hamilton-Norwood II-a | 2 (3.3%) | 0 | 2 (13.3%) | 0.383† |
| Hamilton-Norwood III-v | 5 (8.3%) | 3 (20%) | 2 (13.3%) | 0.383† |
| Hamilton-Norwood IV | 2 (3.3%) | 1 (6.7%) | 1(6.7%) | 0.383† |
| Hamilton-Norwood IV-a | 4 (6.7%) | 2 (13.3%) | 2 (13.3%) | 0.383† |
| Hamilton-Norwood V | 17 (28.3%) | 9 (60%) | 8 (53.3%) | 0.383† |
| Female | ||||
| Ludwig I-4 | 6 (10%) | 1 (6.7%) | 5 (33.3%) | 0.383† |
| Ludwig II-1 | 13 (21.6%) | 8 (53.3%) | 5 (33.3%) | 0.383† |
| Ludwig II-2 | 11 (18.3%) | 6 (40%) | 5 (33.3%) | 0.383† |
| Hair mass index at baseline | 21.52±12.718 | 19.73±11.682 | 23.3±13.639 | 0.281* |
| Total hair count at baseline | 351.6±147.555 | 334.8±108.876 | 368.3±178.496 | 0.385* |
*p-value
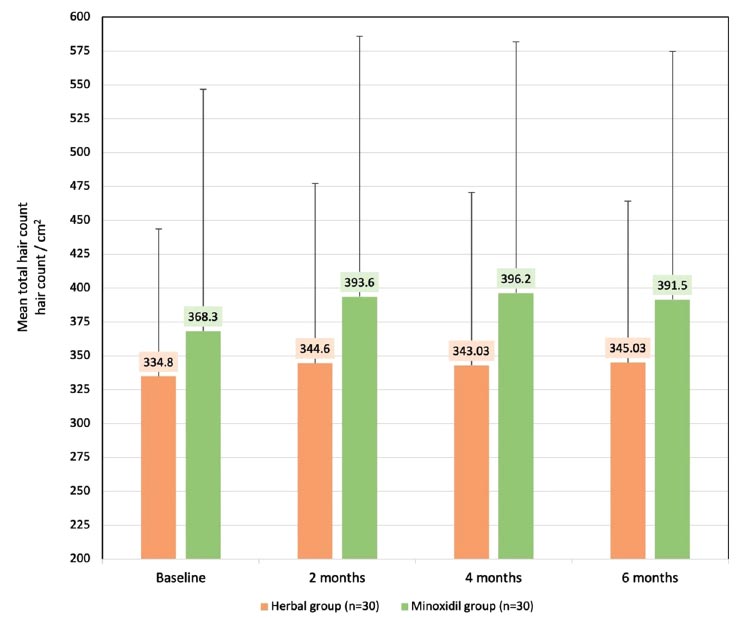
The primary endpoint was the change of mean±SD terminal hair (hair count/cm2) at 8, 16, and 24 weeks from baseline. In the minoxidil group, the total hair count showed a significant increase in mean±SD terminal hair (hair count/cm2) of 368.30±178.49, 393.60±192.23, 396.20±185.73, and 391.50±183.15 (P <0.001) at baseline, 8 weeks, 16 weeks and 24 weeks, respectively. In the herbal group, the total hair count showed a significant increase in mean±SD total hair (hair count/cm2) of 334.80±108.87, 344.60±132.72, 343.03±127.50, and 345.03±119.26 (p<0.001) at baseline, 8 weeks, 16 weeks and 24 weeks, respectively (Fig. 2).
There was a significant increase in the total hair count at 24 weeks (p<0.001, p<0.001) in both groups, respectively, with no statistical difference between the two groups at 8, 16, and 24 weeks (p=0.256, p=0.202 and p=0.250) (Figs. 2 and 3).
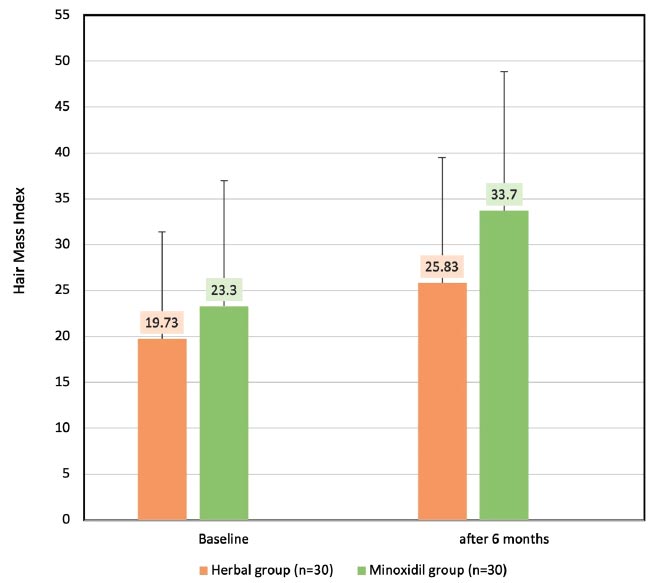
The secondary endpoint was the change of HMI after treatment. In the minoxidil group, the HMI had a significant increase in mean±SD of 23.30±13.63 and 33.7±15.17 (p<0.001) at baseline and 24 weeks, respectively. Meanwhile, the herbal group demonstrated a significant increase in HMI of 19.73±11.68 and 25.83±171.8 (p=0.001) at baseline and 24 weeks, respectively, with no statistical difference between the two groups (p= 0.065) (Fig. 4).
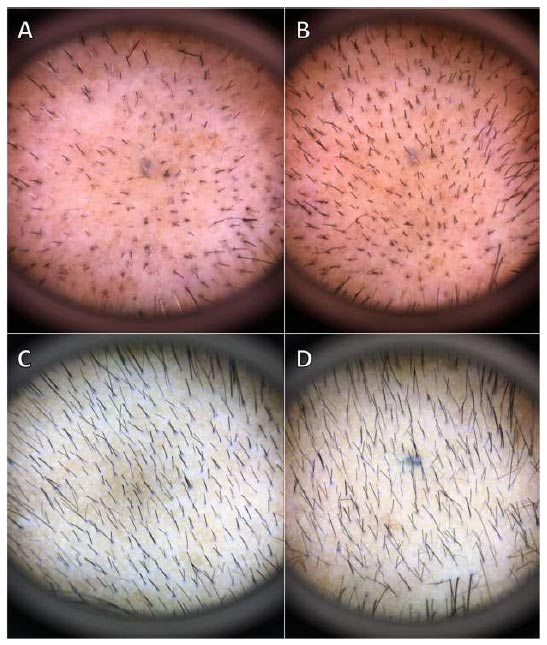
Among the patients in the herbal group, 1.7% had a slight decrease, 10% remained stable, 50% had a slight increase, 30% had a moderate increase, and 8.3% had a significant increase in terms of hair growth. In contrast, 13.3% of the minoxidil group remained stable, while 53.3% had a slight increase, 20% had a moderate increase, and 13.3% had a significant increase in terms of hair growth (Fig. 5). No adverse events were reported in both sexes.
4. DISCUSSION
The pathogenesis of AGA is a result of both genetic predisposition and hormonal effects. The anagen phase is shortened, while the anagen-to-telogen ratio is reduced, and terminal hair gradually turn into vellus hair due to follicular miniaturization. This process is particularly driven by the effect of DHT [4, 6].
The present study demonstrated significant progressive clinical improvement in both groups of patients, starting at 8 weeks up until 16 and 24 weeks, irrespective of sex. The herbal group and minoxidil group had the mean change of total hair count at 24 weeks of 10 hairs/cm2 and 23 hairs/cm2, respectively. Furthermore, both the herbal group and minoxidil group displayed an increase in the hair mass index of 30% and 47% from baseline, respectively. Statistically, there was a non-significant difference in the efficacy between patients whom were treated with the herbal group and those who were treated with the standard 3% minoxidil solution.
*p-value.
Redenyl® is composed of DHQG from the Larix europae wood extract, EGCG from green tea leaves, glycine, zinc, and water. The main active ingredients, DHQG and EGCG, are stabilised polyphenols that target the outer root sheath stem cells and the fibroblasts of the dermal papilla.
EGCG, a major constituent of polyphenols [7, 8], significantly lengthens human hair follicles ex vivo [9]. The mechanism of action for treating AGA includes selective inhibition of 5α-reductase activity, thus enhancing the survival of human keratinocytes and promoting the proliferation of dermal papilla cells (DPCs) via the upregulation of phosphorylated Erk and Akt as well as the increase of the Bcl-2/Bax ratio. It is generally known that Bax causes apoptosis, whereas Bcl-2 has an anti-apoptotic function [9, 10]. By changing the expression of microRNA in human DPCs [9], the detrimental effects of DHT can be minimized, which includes promoted cell death, cell cycle arrest, senescence, and oxidative stress [11]. By encouraging the processes of proliferation and anti-apoptosis on DPCs, the anagen stage is prolonged, which ultimately promotes the development of human hair [9].
Keratin is the main structural protein of hair. According to their amino acid structure, high-glycine/tyrosine proteins (HGTps) are one of the major components [12]. HGTps are composed of glycine, tyrosine, and serine, which are vital in the anagen phase formation of the intermicrofibrillar matrix of the cortex [13]. Moreover, glycine is also involved in hair metabolism [4].
As a catagen inhibitor and regulator of hair development through hedgehog signaling, zinc reduces hair follicle regression and accelerates hair follicle recovery. Furthermore, it also maintains a functional role in hair follicle cycling and morphogenesis [14, 15]. Aiempanakit et al. showed that male AGA patients had lower serum zinc levels than the controls [16], suggesting that zinc may be an essential addition to AGA treatment.
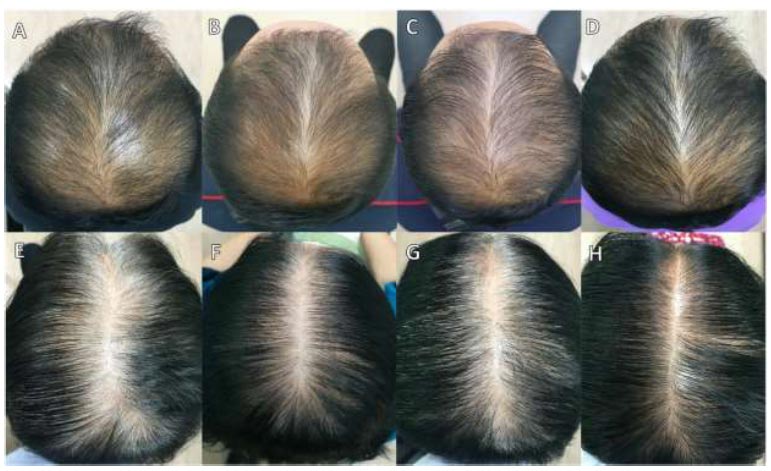
Sfo studies examining the efficacy of Redendyl (R) in treating AGA. Previous literature has not conveyed any similarities or differences between the use of an herbal extract (Redenyl®) and topical minoxidil solution in AGA. With the focus on scrutinizing the efficacy and side effects of the herbal extract compared to minoxidil solution, a study by Olsen et al., in 2002, found that drug-related adverse events were more prevalent in patients treated with 5% minoxidil solution than with 3% minoxidil solution [17]. Considering that patients treated with 3% minoxidil reported minimal side effects during treatment, our study aimed to examine whether patients treated with herbal extract would achieve similarly favorable outcomes. Interestingly, both groups treated with 3% minoxidil solution and the herbal extract reported no drug-related adverse events, further supporting that the herbal extract could be a safe and effective alternative treatment for AGA. In our study, we demonstrated an improvement in AGA following the administration of the herbal extract, which could derive from various mechanisms, including the enhancement of hair regrowth by stimulating proliferative and anti-apoptotic activities on DPCs. The result was a non-significant difference in efficacy and safety when compared to the 3% minoxidil solution, suggesting that this herbal extract could be an alternative treatment for AGA.
CONCLUSION
We found that the herbal extract used in this study can be considered an alternative treatment option for patients who are concerned about the risks and side effects of the current standard treatments for AGA. Our study successfully illustrated significant clinical improvement, comparable to the mainstay treatment of 3% minoxidil solution in a sample group of 60 patients. It is worth noting that the herbal extraction is safe and effective as a potential alternative treatment, as no adverse side effects were reported. However, further studies with a larger sample size of multi-centre trials may be required.
LIST OF ABBREVIATIONS
| AGA | = Androgenetic alopecia |
| DHT | = Dihydrotestosterone |
| VEGF | = Vascular Endothelial Growth Factor |
| DHQG | = Dihydroquercetin-Glucoside |
| DPCs | = Dermal Papilla Cells |
| HMI | = Hair Mass Index |
| HGTps | = High-Glycine/Tyrosine Proteins |
| GMP | = Good Manufacturing Practices |
| COA | = Certificate of Analysis |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the Institutional Review Board of Panyananthaphikkhu Chonprathan Medical Centre under clearance approval number 018/61. This clinical study is registered at www.thaiclinicaltrials.org with identification code TCTR20220927001. Informed consent was provided by all subjects.
HUMAN AND ANIMAL RIGHTS
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or research committee and with the 1975 Declaration of Helsinki, as revised in 2013.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article is available in the Zenodo Repository at doi 10.5281/Zenodo.11114087.


