All published articles of this journal are available on ScienceDirect.
Serum Visfatin Level in Psoriasis Patients: A Case-Control Study
Abstract
Background
Adipokines play imperative roles in the pathogenesis of psoriasis. Among the adipokines, visfatin is attracting more attention in the clinical setting of dermatology.
Objective
The study aims to evaluate the serum visfatin level in psoriasis patients compared to the non-psoriasis individuals.
Material and Methods
This case-control study involved 40 psoriasis patients and 40 non-psoriasis individuals from January to October, 2023, at the Ho Chi Minh City (HCMC) Hospital of Dermato-Venereology. The diagnosis of psoriasis was based on clinical signs and symptoms. Visfatin level was spectrophotometrically measured using an Enzym-Linked Immunosorbent Assay (ELISA) kit. Afterward, data analysis was performed using SPSS version 25.
Results
We recorded a significantly higher visfatin level in the psoriasis group than the controls (49.8 ± 26.04 versus 13.07 ±12.44, p-value <0.001). The cut-off threshold of visfatin level to differentiate psoriasis from non-psoriasis was 21.7 ng/ml with a sensitivity of 90% and a specificity of 85% (AUC = 0.929). We also found a positive correlation between visfatin level and Psoriasis Area and Severity Index (PASI) score (r = 0.704; p <0.001).
Conclusion
Our study indicated the link between serum level of visfatin and psoriasis. Visfatin is a potential biomarker in diagnosing psoriasis and classifying the disease’s severity. In further cohort studies and clinical trials, the adipokine can be validated for its use in psoriasis.
1. INTRODUCTION
Psoriasis, of which prevalence ranges from 0.51% to 11.43% in the population [1], is among the most common problems in dermatology [2]. Psoriasis is a chronic, systemic inflammatory disease characterized by bright red, raised, and scaly patches on the skin, especially on the scalp, elbows, knees, lower back, and joints [3-5]. The increased activity of proinflammatory cytokines leads to characteristic lesions [6], and the lesions themselves stimulate the excretion of inflammatory mediators, resulting in a pathological cycle [7]. Among the mediators, there are adipokines derived from adipocytes. Adipokines act on cellular signals, causing chronic inflammation and many comorbidities [8-10]. Many works described the mechanism of psoriasis and recorded a close relationship between the disease and adipokine activity, including leptin, resistin, and particularly visfatin [10, 8, 11-14].
Visfatin, a pre-B-colony enhancing factor, is predominantly released by visceral fat [15, 16]. Inflammatory activities promote visfatin production from monocytes, neutrophils, and macrophages [17-19]. The intracellular form of visfatin plays a vital role in regulating cellular metabolism and adapting to external stress. Meanwhile, the extracellular form, which is measurable in the serum, greatly impacts both inflammatory and metabolic processes [20]. Visfatin induces the formation of new blood vessels (angiogenesis) through the upregulation of intercellular adhesion molecule 1 (ICAM-1), vascular endothelial molecule 1 (VCAM-1), and vascular endothelial growth factor (VEGF) [16, 21]. The substance also plays a role in the pathogenesis of atherosclerotic plaque formation via reducing nitric oxide (NO) production and increasing oxidative stress [22-24].
Due to its proinflammatory and immunomodulatory effects, literature has concluded the increase of visfatin levels in systemic inflammatory diseases, such as psoriasis [25-31]. Although the correlation between visfatin and psoriasis has been mentioned in many studies, a firm conclusion about the role of visfatin is still under debate due to the difference in study design or population. Therefore, in the clinical setting of Vietnam, our study aims to evaluate the serum level of visfatin in psoriasis patients compared to non-psoriasis individuals so we can further assess the prognostic value of visfatin in the disease.
2. MATERIALS AND METHODS
2.1. Patients Selection
This case-control study was conducted at the Ho Chi Minh (HCMC) Hospital of Dermato-Venereology from January to October, 2023. For the selection of patients and data collection, the study protocol and national ethics disciplines were followed. All patients who participated in the study willingly signed the informed consent form after being carefully consulted.
The diagnosis of psoriasis was based on clinical signs and symptoms, and we used the PASI score to evaluate the severity of psoriasis. The patient's history was based on medical records or current prescriptions. The exclusion criteria for the case and control groups were people with a prior diagnosis of diabetes mellitus, current infections, malignancies, or any autoimmune disorders. The patient group did not receive systemic psoriasis treatment and had ceased topical treatment for four weeks.
2.2. Study Design
According to a previous study by Okan et al. (2016), we calculated the minimum sample size needed for each group using the formula as follows:
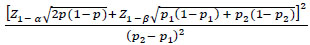 |
It was used with a power of 95% and a type I error of 5%. By that, we intended to enroll 40 psoriasis patients and 40 non-psoriasis individuals as controls.
A pack-year unit was used to approach a more accurate representation of smoking exposure by quantifying the amount of cigarette smoking a person has done over time. One pack-year is equivalent to smoking 20 cigarettes (one pack) per day for one year.
Mean arterial pressure (MAP) was calculated based on systolic and diastolic pressure using the following formula:
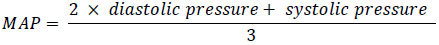 |
The primary outcome of this study was the serum visfatin level in psoriasis patients. Serum visfatin levels were determined using an Enzyme-Linked Immunosorbent Assay (ELISA) (from ThermoFisher, USA) following the manufacturer's protocol using a venous blood sample in an Ethylene Diamine Tetra Acetic (EDTA) tube. Blood samples were obtained in the morning after a 12-hour fast and sent to the Center for Molecular Biomedicine (University of Medicine and Pharmacy in Ho Chi Minh City). Lipid profiles were assessed at the laboratory department of the study hospital. We defined metabolic syndrome based on the a-NCEP criteria as described previously [17].
2.3. Statistical Analysis
We used Microsoft Excel and SPSS software to process data and perform statistical analyses. The Chi-squared test evaluated nominal variables. Continuous variables were compared using the Student's t-test and analyzed using Pearson's correlation. Receiver Operating Curve (ROC) analysis identified the cut-off value of visfatin between groups. P-value <0.05 was considered statistically significant.
3. RESULTS
3.1. Demographics and Baseline Characteristics
We enrolled 40 patients with psoriasis and 40 non-psoriasis controls in the study. Male participants accounted for 55% of the studied population. The socio-demographics and clinical features of psoriasis patients and controls are included in Table 1. The average age of the patients in the study was 42.18 ± 15.17. Metabolic syndrome was present in 40% of psoriasis patients. There were no significant socio-demographic differences between groups. The mean value of the PASI score was 15.26 ± 7,43.
3.2. Serum Visfatin Levels of Patient and Control Groups
The visfatin level in psoriasis patients had a mean value of 49.8 ± 26.04 ng/ml, which was significantly higher than the controls (p<0.001) (Table 2).
| Socio-demographics and Baseline Characteristics |
Psoriasis (N = 40) |
Control (N = 40) |
p-value |
|---|---|---|---|
| Age, years (mean ± SD) |
42.18 ± 15.17 | 42.9 ± 14.9 | 0.93* |
| Sex, male (n; %) | 22 (55%) | 22 (55%) | 1 † |
| Smoking status (n; %) | 16; 40% | 8; 20% | 0.106 † |
| Alcohol consumption (n; %) | 21; 52.5% | 20; 50% | 0.32 † |
| BMI, kg/m2 (mean ± SD) | 23.29 ± 3.76 | 22.98 ± 2.31 | 0.82* |
| Waist, meter (mean ± SD) | 0.85 ± 0.09 | 0.80 ± 0.08 | 0.125* |
| Mean arterial pressure, mmHg (mean±SD) | 95.09 ± 7.97 | 96.83 ± 7.76 | 0.19* |
| Total cholesterol (mmol/L) (mean±SD) | 4.91 ± 1.04 | 4.90 ± 1.03 | 0.07* |
| HDL-c, mmol/L (mean ± SD) | 1.26 ± 0.3 | 1.37 ± 0.32 | 0.14* |
| LDL-c, mmol/L (mean ± SD) | 2.78 ± 0.99 | 2.81 ± 0.79 | 0.64* |
| Glucose, mmol/L (mean ± SD) | 5.63 ± 0.97 | 5.58 ± 0.92 | 0.86* |
| Metabolic syndrome (n; %) | 16; 40% | 20; 55.6% | 0.369 † |
| PASI, points (mean ±SD) | 15.26±7.43 | - | - |
| - | Psoriasis | Controls | p-value |
|---|---|---|---|
| Visfatin, ng/ml (mean ± SD) | 49.8 ± 26.04 | 13.07 ± 12.44 | p <0.001* |
| - | r | p-value* |
|---|---|---|
| Age, years | -0.24 | 0.141 |
| Pack-year | -0.59 | 0.016 |
| Waist, cm | 0.29 | 0.069 |
| Mean blood pressure, mmHg | -0.36 | 0.023 |
| Glucose, mmol/L | 0.16 | 0.34 |
| LDL-C (mmol/L) | -0.28 | 0.085 |
| HDL-C (mmol/L) | 0.16 | 0.313 |
| Cholesterol TP (mmol/L) | -0.134 | 0.41 |
| Duration of disease | 0.26 | 0.107 |
| PASI | 0.704 | <0.001 |
3.3. Cut-off Value of Visfatin Levels
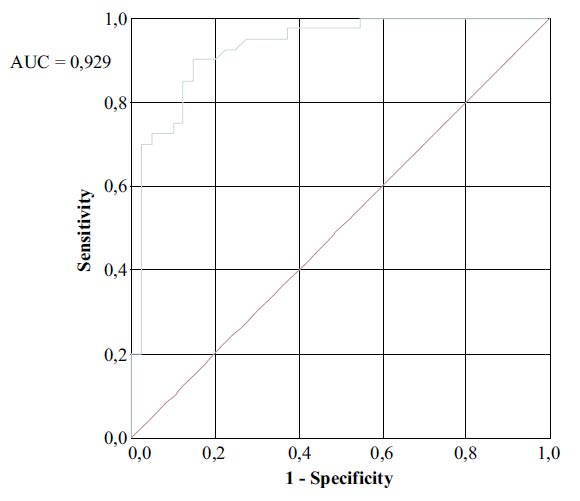
The ROC curve of visfatin level in the studied population showed that visfatin level could differentiate between psoriasis and non-psoriasis with AUC = 0,929; the cut-off level was 21.7 ng/ml (sensitivity: 90%; specificity: 85%) (Fig. 1).
Positive correlations were noted between the serum visfatin level and PASI scores (p <0.001), pack-year index (p = 0.016), and mean blood pressure (p = 0.023) (Table 3).
4. DISCUSSION
Many authors reported similar results to our findings [27-29, 32]. In a meta-analysis by Q. Zou et al. (2021), the authors reviewed all the case-control studies and found highly significant visfatin levels in psoriasis patients [10]. Our results showed no significant difference in socio-demographic and clinical features between patients and control groups [33-37]. We observed that the serum visfatin level in the psoriasis group was 49.8 ± 26.04 ng/ml, which was significantly higher than that in the control group (13.07 ± 12.44 ng/ml).
We noticed high proportions of smoking, alcohol consumption, and metabolic syndrome in the psoriasis group, indicating unhealthy lifestyles in these patients and setting goals for better management of the disease [38-41]. Visfatin is an adipokine derived from adipocytes, but we only observed the difference in visfatin levels, while all other metabolic profiles were not significantly different. The results suggested a direct link between visfatin and psoriasis, as described previously by Q. Zou et al. (2021) [10]. Moreover, we also examined the potential of visfatin in distinguishing psoriasis from non-psoriasis patients. By building the ROC curve of the visfatin level, we identified a cut-off at 21.9 ng/ml with an Area Under the Curve (AUC) of 0.929. A. E. El-Rifaie et al. (2022) reported a 9.035 ng/mL cut-off with an AUC of 0.948 [29]. The serum visfatin level can vary between distinct populations, influenced by many factors, such as anthropology or nutrition [42-45]. Although the cut-off from our result was relatively high, the AUCs from both studies showed consistent values. Nevertheless, validation studies in different dermatological diseases are needed to confirm the cut-off for clinical purposes.
Significant correlations were found between serum visfatin, pack-year index, mean blood pressure, and PASI score. D. Dimov et al. (2019) [46] reported a high visfatin level in the smoking group [46]; however, we observed a negative correlation between visfatin level and pack-year index. Many studies indicated that visfatin is often associated with mechanisms that increase blood pressure through its effects on inflammation and endothelial dysfunction [47-49]. In contrast, our study observed a lower mean blood pressure associated with a higher visfatin concentration. We believe that visfatin may increase the production of Nitro Oxide (NO), which dilates blood vessels, as mentioned in some previous studies. Due to the conflicting results, further research might propose an answer.
We found a positive correlation to visfatin level for the PASI score, which was consistent with previous studies [10, 28, 29, 39, 50-52]. In addition, a study by Ismail et al. also reported that the visfatin level in patients with severe psoriasis was significantly higher than in the group with mild-moderate psoriasis [40]. Therefore, we concluded the correlation between visfatin level and the disease's severity. It is hypothesized that visfatin contributes to the increase of inflammatory and immune responses via monocyte stimulation, which induces large amounts of IL-1, TNF-α, and IL-6 [51]. On the other hand, in the immunopathogenesis of psoriasis, skin lesions are stimulated directly to increase serum visfatin levels [21]. Using an in-vitro model on murine, N. Kanda et al. (2011) described that visfatin levels could aggregate the inflammatory state by enhancing the activity of chemokine CXC Motif Ligands (CXCL), such as CXCL 8, CXCL 10, and chemokine ligand (CCL20). Besides psoriasis, visfatin levels also escalate in other systemic inflammatory diseases, such as diabetes mellitus type 2 and atherosclerosis [53-58]. A synthesis of the above observations indicates that high visfatin concentration plays a pivotal role in activating the systemic inflammatory response, which exacerbates the severity of psoriasis. This fascinating mechanism proves the specificity of visfatin to psoriasis severity, which suggests a specific visfatin cut-off level to classify the severity of psoriasis, along with the PASI score. On the other hand, visfatin levels could be a promising biomarker for assessing treatment responses in cohort studies.
One major limitation of the study is the relatively small sample size; in a study with a larger sample size, we could obtain a cut-off with better sensitivity. The second one is that we could not simultaneously evaluate other adipokines and cytokines to highlight the importance of visfatin in psoriasis. Therefore, we propose further studies that could overcome these gaps to confirm the capability of visfatin for clinical purposes [59, 61].
CONCLUSION
Serum visfatin could be a potential biomarker to diagnose and classify psoriasis based on the positive correlation between its level and PASI score. Further research in a larger population is needed to validate the cut-off threshold of visfatin and elicit its role in psoriasis pathogenesis.
LIST OF ABBREVIATIONS
| AUC | = Area Under the ROC Curve |
| BMI | = Body Mass Index |
| CXCL | = Chemokine (C-X-C motif) Ligand |
| CCL | = Chemokine Ligand |
| ELISA | = Enzyme-Linked Immunosorbent Assay |
| EDTA | = Ethylene Diamine Tetra Acetic |
| HCMC | = Ho Chi Minh City |
| HDL-C | = High-Density Lipoprotein Cholesterol |
| IL | = Interleukine |
| LDL-C | = Low-Density Lipoprotein Cholesterol |
| MAP | = Mean Arterial Pressure |
| OR | = Odds ratio |
| ROC | = Receiver Operating Curve |
| PASI | = Psoriasis Area and Severity Index |
| TNF-α | = Tumor Necrosis Factor α |
| SD | = Standard Deviation |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The Ethics Committee of the Pham Ngoc Thach University of Medicine has reviewed and approved our study (code: 739/TĐHYKPNT-HĐĐĐ).
HUMAN AND ANIMAL RIGHTS
All human research procedures followed the ethical standards of the committee responsible for human experimentation (institutional and national) and with the Helsinki Declaration of 1975, revised in 2013.
CONSENT FOR PUBLICATION
Informed written consent was taken from all the patients when they were enrolled.


