All published articles of this journal are available on ScienceDirect.
Evaluating the Role of Topical Immunomodulators for Molluscum Contagiosum: A Review
Abstract
Background
Molluscum contagiosum is a common skin infection caused by the molluscum contagiosum virus. The condition can persist for years due to viral immune evasion mechanisms, leading to significant physical and psychosocial impacts.
Objective
This review aimed to evaluate topical immunomodulators for the treatment of molluscum contagiosum, focusing on their mechanisms, administration methods, clinical safety, and efficacy.
Methods and Results
A literature search conducted using Pubmed, Google Scholar, and Medline identified five topical immune-stimulating therapies: tretinoin, adapalene, diphencyprone, imiquimod, and berdazimer sodium. While imiquimod is no longer recommended and larger-scale studies are warranted to assess the role of tretinoin, adapalene, and diphencyprone; berdazimer sodium has received FDA approval for molluscum contagiosum treatment.
Conclusion
The mechanisms underlying topical immunomodulators remain elusive, and long-term comprehensive studies are required to evaluate their effectiveness across diverse presentations of molluscum contagiosum.
1. INTRODUCTION
Molluscum contagiosum (MC) is a common skin condition that predominantly affects children [1] and ranks among the top 50 most prevalent diseases worldwide [2]. It manifests as characteristic raised skin lesions that occur almost anywhere on the body [3]. MC is caused by molluscum contagiosum virus (MCV), a virus within the Poxviridae family [4]. The immune system plays a predominant role in the clearance of MCV, as is typical with viral infections. However, MCV utilizes several mechanisms to evade the immune response [5], resulting in skin lesions that can persist for several years [1] and cause even more extensive disease in immuno- compromised patients. Mounting a vigorous immune reaction is essential in resolving MCV infection. This review aims to evaluate topical treatments that stimulate the immune response in managing MC.
1.1. Background
The viral etiology of MC was first described in 1905 [6]. MCV is a member of the Poxviradae family, characterized by enveloped double-stranded DNA viruses. Several other poxviruses cause human disease; these include variola virus, the causative agent of smallpox, which has been eradicated worldwide, and mpox, the causative agent of Monkeypox, which was responsible for a recent outbreak [4]. Although MCV has not triggered large outbreaks [7], it remains a common cause of infectious disease across the globe.
MCV is transmitted by nonsexual or sexual direct contact with infected skin or contaminated fomites [8]. It primarily affects children under 14 years old, showing minimal variation in prevalence between males and females [1]. MC is also frequent in immunocompromised individuals, often affecting those with human immuno- deficiency virus (HIV), acquired immunodeficiency syndrome (AIDS), primary immunodeficiency, and iatrogenic immunosuppression [9]. Recent retrospective studies identified that patients with atopic dermatitis (AD) have a higher likelihood of MC [10, 11], likely due to disruption of the skin barrier and immune regulation [12]. Furthermore, MC has been recognized as a sexually-transmitted disease that affects sexually active adolescents and adults [13].
Following MCV infection, symptoms of MC generally develop within a span of 14 days to 6 months [14]. MC typically presents as 2 to 5 mm firm, dome-shaped, shiny, skin-colored papules with central umbilication [9]. These papules may appear individually or as clusters in areas of exposed skin, including the trunk, extremities, face, genitals, and intertriginous regions [9], usually sparing the palms, soles, and oral mucosa [15]. The lesions may be pruritic [9]. The timeframe for resolution varies, and lesions have been reported to persist for several months to five years [1]. A more recent study reported a mean lesion resolution time of 13.3 months [16]. This prolonged resolution period is attributed to MCV’s ability to escape the immune response by remaining within the epidermis throughout the disease course [12, 17] and producing proteins to bypass immune surveillance [5]. Following this period of immune evasion, an inflammatory response termed the beginning of the end (BOTE) precedes the resolution of MCV infection and may present clinically as an inflamed lesion [18]. It is important to note that immunocompromised individuals with MC tend to develop more extensive disease with increased quantities, distribution, and size of skin lesions that do not demonstrate spontaneous resolution [19, 20].
MCV localizes within the epidermis throughout its disease course [12, 17]. Its cell entry mechanisms are not well characterized due to the difficulty of propagating the virus in tissue culture systems [21, 22]. Initially, MCV proliferates within the cytoplasm of keratinocytes in the stratum basale, where it induces overexpression of epidermal growth factor receptor (EGFR) [23, 24]. Infected keratinocytes then differentiate and expand apically. Characteristic molluscum bodies, which serve as the sites of viral assembly within the cytoplasm, are observed within the stratum spinosum and stratum granulosum layers [12]. The stratum corneum disintegrates as the molluscum bodies enlarge [12], releasing infectious virions through a keratinized tunnel below the central umbilication of the lesion [25].
The diagnosis of MC is made clinically, although a biopsy can be performed for confirmation. Histopathologic analysis reveals hyperkeratosis and characteristic molluscum bodies, also known as Henderson-Paterson intracytoplasmic eosinophilic inclusion bodies [9]. While diagnosing MC is relatively straightforward, the need for treatment in immunocompetent patients is highly debated. There is a lack of consensus on awaiting the natural resolution of typical lesions versus actively treating MC to accelerate the resolution process [26].
Untreated MC can lead to various consequences, including persistent discomfort and pruritus; anxiety and social withdrawal stemming from lesion appearance or transmission concerns; local skin irritation resulting from secondary infection or attempted self-treatment; and continued transmission through self-inoculation or to others, posing a particular risk to immunocompromised individuals who may experience more severe mani- festations of MC [1, 8, 16, 26].
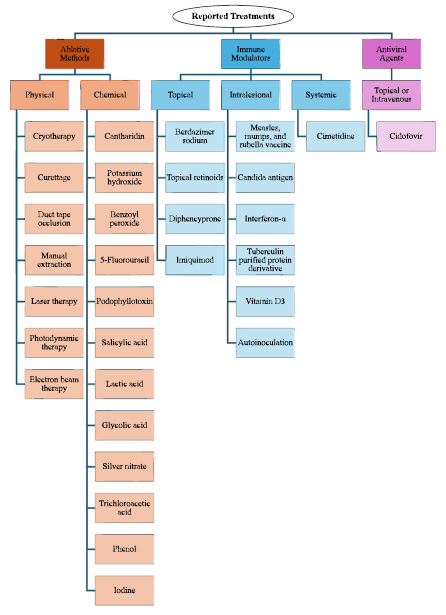
The best treatment approach for MC remains uncertain. In fact, there was no United States (US) Food and Drug Administration (FDA)-approved treatment for MC until July 2023 [27]. Treatment methods for immuno- competent patients generally fall into three categories: ablative methods, immunomodulating therapies, and antiviral agents (Fig. 1) [12, 26, 28, 29].
1.2. Immune Response
The skin serves as the first line of defense against pathogens, playing a vital role in the immune system. Upon disruption of the epidermal barrier, cells of the innate immune system are rapidly activated [30]. Macrophages, dendritic cells, and natural killer (NK) cells are the main constituents of this process, with keratinocytes and Langerhans cells fundamental to innate immunity within the epidermis [31]. These innate immune cells detect pathogen-associated molecular patterns (PAMPs) on invading microbes through their pathogen recognition receptors (PRRs), initiating a signal transduction cascade [17, 32]. The downstream effect is the activation of transcription factors such as nuclear factor kappa light chain enhancer of activated B cells (NF-κB) and interferon regulatory factor (IRF), which upregulate various immune mediators essential for pathogen detection and clearance [33, 34]. These mediators, which include antimicrobial peptides, inter- leukins, Type I interferons, tumor necrosis factor (TNF), reactive oxygen species (ROS), and nitric oxide (NO), are essential for the innate immune response [31, 34]. Dendritic cells also serve as intermediaries between the innate and adaptive immune system, presenting signals such as major histocompatibility complex (MHC) molecules to activate adaptive cells and amplify the immune reaction [31]. The interactions and downstream effects of dendritic cell subsets are associated with the spontaneous resolution of MC [35].
Despite its high viral load, MCV does not provoke a substantial immune response for months to years [33]. A recent review outlined a myriad of viral proteins postulated to contribute to MCV’s immune evasion [5]. These proteins function as inhibitors of various immune pathways. Notably, proteins MC005, MC008, MC132, MC159, MC160, and MC163 impede NF-κB activation, with MC159 and MC160 also preventing IRF-3 activity [5, 33, 34, 36-41]. Beyond transcription factors, MCV-encoded proteins target other immune mediators; MC53 and MC54 neutralize interleukin-18 [42], MC80 inhibits the action of MHC I [43, 44], and MC148 dampens immune cell chemotaxis and recruitment [5, 45]. Additionally, MCV produces several proteins that promote its continued replication. MC159 and MC163 block apoptosis signaling pathways, and MC66 is a glutathione peroxidase homolog that specifically inhibits apoptosis induced by oxidative stress or UV radiation [5, 22, 46, 47]. The promotion of cellular proliferation by MC007 further contributes to the development of the persistent skin lesions characteristic of MCV [5, 48].
It is evident that MCV employs diverse strategies to attenuate the immune response. Thus, bolstering the immune system presents a promising therapeutic approach for resolving MC. To explore this further, we conducted a literature review focusing on topical immunomodulators that may enhance the immune response against MCV. These therapies are minimally invasive and offer advantages over ablative methods known for their risks of pain and scarring [12]. By examining a spectrum of both older and newer topical immune-stimulating therapies, our investigation aims to provide valuable insights that can shape future treatment modalities for MC.
2. MATERIALS AND METHODS
A literature search was conducted at Pubmed, Google Scholar, and Medline, with the keywords “molluscum contagiosum,” “imiquimod,” ”diphencyprone,” “diphenyl- cyclopropenone,” “retinoid,” “tretinoin,” “adapalene,” “tazarotene,” “trifarotene,” “berdazimer,” “nitric oxide,” “SB206,” “immune,” “immunotherapy,” “mechanism,” and “dermatology.” Extensive review of the bibliography of each included article was conducted.
Inclusion Criteria: Original research, review articles, and case studies written in English that examined the use of topical immunomodulators for MC, encompassing all patient age groups, immune statuses, comorbid conditions, and MC presentations, published before May 15, 2024.
Exclusion Criteria: This review did not evaluate ablative therapies, antiviral therapies, and non-topical immunotherapies for MC.
3. RESULTS
3.1. Topical Retinoids
Topical retinoids serve as versatile treatments for a wide array of dermatologic conditions. Retinoids exhibit structural or functional similarities to Vitamin A or are derived from it [49]. Vitamin A and its metabolites play an essential role in epithelial cell differentiation, immune function, embryonic development, and vision [49-51]. Retinoids bind to subtypes of two nuclear receptors: retinoic acid receptors (RAR) and retinoid X receptors (RXR) [49, 52]. Upon binding to nuclear receptors, retinoids form complexes that function as transcription factors, thereby regulating the expression of hundreds of gene products [49, 52]. The direct and indirect effects of retinoids lead to the inhibition of keratinocyte proliferation and modulation of immune responses [52]. While retinoids downregulate various immune mediators and transcription pathways, they are also postulated to enhance the secretion of specific interleukins, promote dendritic cell migration, and activate Langerhans and NK cells within the innate immune system [52]. Concurrently, retinoids contribute to regulating T-cell function within the adaptive immune system [52]. While the precise mechanism of action remains elusive, the broad immunomodulatory effects and control of keratinocyte proliferation by retinoids have demonstrated clinical utility in managing various dermatologic conditions. Two topical retinoids, tretinoin and adapalene, have been reported in the treatment of MC (Figs. 2-3).
Chemical structure of tretinoin.
PubChem Identifier: CID 444795
URL: https://pubchem.ncbi.nlm.nih.gov/compound/444795#sec
tion=2D-Structure
Chemical structure of adapalene.
PubChem Identifier: CID 60164
URL: https://pubchem.ncbi.nlm.nih.gov/compound/60164#sec
tion=2D-Structure
Tretinoin, the first topical retinoid developed, is FDA-approved for several indications, including acne vulgaris [50]. It is available in multiple creams, lotion, gel, and microsphere gel formulations [53]. Despite two compa- rative studies assessing the safety and efficacy of 0.05% tretinoin cream for MC in children, their limited sample sizes rendered the evidence insufficient to support its use, according to a comprehensive Cochrane review that evaluated randomized clinical trials (RCTs) across the globe from 1990-2015 [26, 54]. In the interim, two studies were conducted to evaluate 0.05% tretinoin cream for treating MC in both children and adults; however, these studies were also limited in scale [55, 56].
Adapalene, another FDA-approved topical retinoid for acne vulgaris, is generally considered better tolerated than tretinoin and is offered in several cream, gel, and lotion formulations [50, 53]. One case report described the case of a child with AD and MC who responded to adapalene 1% cream within four weeks [57], while another case report documented the case of two children with periocular MC who experienced rapid resolution with twice-daily application of adapalene 0.1% gel [58]. Additionally, three small studies, including a prospective study and two comparative investigations, examined the effectiveness of adapalene 0.1% in treating MC in children and noted some lesion clearance as well as adverse effects [59-61]. Topical retinoids have been associated with adverse effects such as photosensitivity and application site reactions, including dryness, peeling, erythema, and pruritus [49, 62]. No published studies were found investigating the effectiveness of other topical retinoids, including tazarotene and trifarotene, in MC treatment.
3.2. Diphencyprone
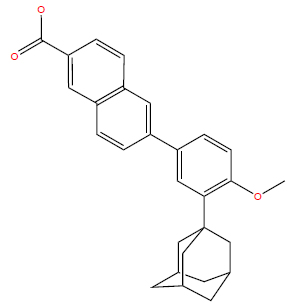
Diphencyprone, or diphenylcyclopropenone (DPCP), is a synthetic contact sensitizer that elicits a Type IV delayed hypersensitivity reaction (Fig. 4). This inflammatory reaction is believed to redirect the immune system, assisting in the management of certain autoimmune, infectious, and neoplastic conditions [63, 64]. In the treat- ment of cutaneous viral infections, contact sensitizers are postulated to bind to antigens associated with the infection, facilitating their recognition by Langerhans, dendritic, and NK cells, thereby prompting an immune response [63, 65]. Additionally, contact sensitizers modulate local immune mediators, including specific interleukins, and restore equilibrium among adaptive T-cells [66, 67]. These insights suggest the therapeutic potential of DPCP across various conditions, although the precise mechanisms governing its effects remain unclear.
No published studies were found investigating the efficacy of contact sensitizers other than DPCP in the treatment of MC. DPCP is a suitable contact sensitizer because it is non-mutagenic and has a low potential of inducing cross-sensitivity to substances commonly encountered in the environment [68]. DPCP has been utilized since the late 1970s for off-label treatment of alopecia areata, human papillomavirus warts, and cutaneous melanoma metastases [69]. The FDA recently approved DPCP as a bulk drug substance that can be used for compound topical medications [70]. DPCP is susceptible to degradation when exposed to heat and ultraviolet (UV) light; this is partially counteracted by its conventional solvent, acetone [71]. The contact sensitization treatment approach typically begins with the application of higher concentrations of DPCP, ranging from 1-4% to an inconspicuous area of the skin to induce sensitization, followed by weekly or biweekly treatments with lower DPCP concentrations targeting the areas of the skin to be treated [69]. Common adverse effects associated with DPCP include contact dermatitis and lymphadenopathy, while less frequent adverse effects include urticaria, pigment changes, vitiligo, and erythema multiforme [63].
Chemical structure of diphencyprone.
PubChem Identifier: CID 65057
URL: https://pubchem.ncbi.nlm.nih.gov/compound/65057#sec
tion=2D-Structure
Several studies have evaluated the effectiveness of DPCP in treating MC. One case report detailed a 1% DPCP acetone solution applied to the right shoulder for sensitization, followed by weekly or biweekly applications of 0.05% to 0.1% DPCP acetone to the left shoulder. This treatment resulted in near complete clearance of genital MC lesions in a 3-year-old with a history of AD within 8 weeks, although spontaneous resolution could not be ruled out [72]. Another case involved a patient with HIV and over 100 generalized MC lesions. Sensitization was performed with a 2% DPCP solution, followed by 0.001% to 2.0% DPCP solution applications to 10% of the lesions at weekly or biweekly intervals. The eyelid lesions cleared within 2 months, and the remaining lesions resolved within an unspecified period of time [68].
A larger study of 23 children used a 0.1% DPCP solution for sensitization, followed by daily applications of 0.01% DPCP to MC lesions. Nine patients showed resolution of all lesions within 6 weeks, three patients showed resolution by 20 weeks, and the remaining were lost to follow-up for reasons unrelated to adverse effects. Sensitization alone cleared lesions in two patients. A limitation was that all DPCP applications were performed at home [73]. Another study of 22 children involved sensitization with 0.5% DPCP, followed by weekly applications of 0.0001% DPCP to MC lesions, gradually titrated to a maximum of 0.1% as needed to sustain erythema and pruritus. Fourteen children achieved complete lesion clearance within 5 weeks, but four dropped out due to adverse effects. Prominent treatment responses were observed in areas other than the application sites [74].
In a recent case-controlled study involving 24 patients of all ages with MC, participants were randomized into either a control group receiving normal saline or a treatment group receiving weekly topical DPCP. Adults were sensitized with a 1% DPCP solution, while children were sensitized with a 0.5% solution. Following sensitization, lesions were treated with 0.0001% DPCP solution weekly. The DPCP concentration was adjusted based on individual reactions, with some patients receiving up to 0.1% to maintain erythema and irritation. In the treatment group, 8 of 12 patients achieved complete lesion clearance, while the remaining 4 showed partial response. No patients in the control group experienced lesion resolution within 12 weeks. In those with complete response, no lesion recurrence was observed at the 3-month follow-up [75].
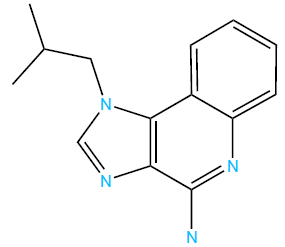
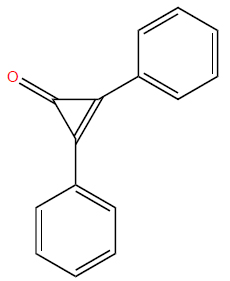
Chemical structure of imiquimod.
PubChem Identifier: CID 57469
URL: https://pubchem.ncbi.nlm.nih.gov/compound/57469#sec
tion=2D-Structure
3.3. Imiquimod
Imiquimod, an imidazoquinoline amine (Fig. 5), activates toll-like receptors (TLRs) 7 and 8, which are specific PRRs present in innate immune cells, including plasmacytoid dendritic cells [76]. This activation triggers the NF-κB pathway, resulting in the expression of diverse immune mediators. Notably, imiquimod can also activate NF-κB through pathways independent of toll-like receptors [76, 77]. Imiquimod’s mechanism includes inducing interferon-α, interferon-γ, TNF-α, and various interleukins, thereby stimulating both innate and adaptive immunity [76, 78, 79]. Moreover, imiquimod is postulated to enhance Langerhans cell migration [80], diminish angiogenesis [81], and induce cell apoptosis at higher concentrations [77]. This multifaceted mechanism of action underscores its therapeutic potential across a range of conditions.
Imiquimod is available in cream formulations with concentrations of 2.5%, 3.75%, and 5% [82]. In the United States, imiquimod is FDA-approved to treat actinic keratosis, external genital warts, and superficial basal cell carcinoma [76, 78]. However, it has been used off-label in the treatment of numerous dermatologic conditions, including MC [76, 78, 83]. In fact, imiquimod is one of the most well-studied treatments for MC [84].
Although imiquimod demonstrated some utility in early unpublished and published studies with limited sample sizes [26, 85-87], two large FDA-requested RCTs and one pharmacokinetics study failed to demonstrate imiquimod’s effectiveness in 2006 [88-90]. Furthermore, these studies raised concerns about application site reactions and less common adverse effects such as flu-like symptoms [91]. Alarmingly, neither RCT was published [91]. A recent Cochrane review concluded that imiquimod 5% cream was no more effective than placebo in curing MC, and indicated imiquimod poses a greater risk of harm compared with placebo due to the proportion of participants experiencing application site reactions [26, 84]. These application site reactions include pain, erythema, pruritus, ulceration, burning, scaling, and pigment changes [26]. Thus, imiquimod is neither safe nor effective for MC treatment [84, 89]. The ineffectiveness of imiquimod may be partially explained by the ability of MCV to produce multiple proteins that prevent NF-κB activation, one of imiquimod’s primary mechanisms of action [76].
3.4. Berdazimer Sodium
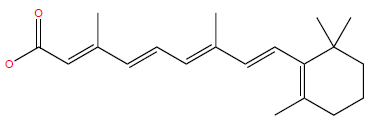
Berdazimer sodium is a macromolecule that releases NO upon exposure to a proton donor (Fig. 6). NO, produced by various cells, including keratinocytes, plays a vital role in pathogen defense by functioning as a signaling molecule that enhances the growth and activity of immune cells [92, 93]. At higher concentrations, NO participates in chemical reactions that disrupt pathogen DNA, proteins, and lipids, thereby contributing to their damage and neutralization [92]. In vitro studies have demonstrated that berdazimer sodium inhibits poxvirus replication and reduces the downstream expression of MC160 [94], one of MCV’s immune evasion proteins postulated to decrease NF-κB and IRF-3 activity [5]. This suggests that ber- dazimer sodium may exert direct effects on MCV through protein nitrosylation and NF-κB modulation [94]. However, further research is needed to fully understand the mechanism of action of berdazimer sodium and the complex pathways through which NO modulates the immune system.
Berdazimer topical gel 10.3% received FDA approval in January 2024 for treating MC in patients aged 1 year and older [95, 96]. This product comprises both a berdazimer sodium gel and a hydrogel, which serves as the proton donor [97]. Patients and their caregivers are instructed to blend equal amounts of the two gels and apply the resulting mixture directly onto MC lesions once daily at home for a duration of up to 12 weeks [95, 96]. Of note, berdazimer topical gel should not be applied on or near mucosal areas [95, 96]. Commonly reported adverse effects include application site reactions characterized by pain, erythema, pruritus, exfoliation, dermatitis, and swelling [95, 96]. Less frequently observed adverse effects include fever, vomiting, upper respiratory tract infection, and application site issues, including erosion, discolo- ration, vesicles, or infection [95, 96].
The berdazimer sodium in molluscum patients with lesions (B-SIMPLE)4 study, a phase III, multicenter, randomized, double-blind trial, assessed the safety and efficacy of berdazimer topical gel, 10.3%, in patients with MC [97]. By week 12, 32% of patients treated with berdazimer topical gel had complete lesion resolution, compared to 20% in the vehicle group [97]. Additionally, 43% of the treatment group experienced a 90% or greater reduction in baseline MC lesions, compared to 24% in the vehicle group [97]. These results were statistically significant and consistent with findings from the preceding B-SIMPLE1 and B-SIMPLE2 phase III trials [97]. The resolution of MC lesions with berdazimer sodium may be attributed to its promotion of BOTE [98]. A systematic review and meta-analysis of the three B-SIMPLE trials and a phase II RCT [99] supported the effectiveness of berdazimer topical gel, demonstrating higher rates of complete lesion clearance and reduced incidence of lesion scarring in patients treated with berdazimer sodium compared to the vehicle [100]. An integrated analysis of the three B-SIMPLE trials further confirmed the statistically favorable efficacy of berdazimer topical gel 10.3% across most subgroups. However, some subgroups, such as Black or African American patients, did not show statistically significant complete clearance rates at week 12, likely due to their limited representation in these trials [101]. This finding reflects the limitations that may impact generalizability of the results. The trials predominantly enrolled white patients and excluded immunocompromised individuals or those with sexually transmitted MC [97]. Furthermore, there has been no investigation into the long-term safety and efficacy beyond the 12-week mark, nor has the effectiveness of berdazimer sodium been compared with other common MC therapies or examined in combination with them [29, 97].
4. DISCUSSION
MC is a common skin condition with a reported prevalence among children ranging from 5.1% to 11.5% [1]. It is caused by MCV, which infiltrates the epidermis and utilizes various immune evasion strategies to proliferate, resulting in characteristic skin-colored papules that can persist for years [9]. The decision to actively treat typical MC lesions in immunocompetent patients is highly debated. Although awaiting natural resolution is an option, untreated MC can impose significant physical and psychosocial burdens on patients and caregivers, exacerbated by the heightened risk of transmission and persistent irritation from self-administered home remedies or unregulated over-the-counter agents [8, 102, 103]. Therefore, considering treatment options is crucial. Common ablative treatments such as curettage and cryotherapy may not be well-tolerated, particularly in patients with multiple MC lesions and children, who make up the majority of MC cases [12]. Topical immunomodulators offer a minimally-invasive alternative that strengthens the immune response – a particularly promising approach given MCV’s evasion tactics. Moreover, these treatments bypass the systemic side effects of oral immunotherapies and are increasingly accessible as new agents receive FDA approval.
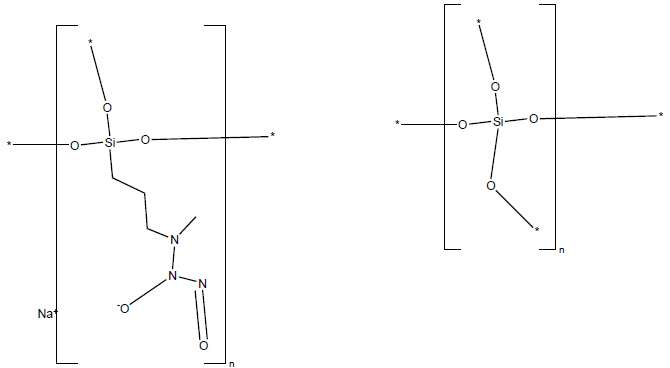
Chemical structure of berdazimer sodium.
PubChem Identifier: SID 472406278
URL: https://pubchem.ncbi.nlm.nih.gov/substance/472406278#section=2D-Structure
Five topical immune-stimulating therapies were identified for the treatment of MC: tretinoin, adapalene, DPCP, imiquimod, and berdazimer sodium (Fig. 7). Among these, berdazimer sodium has demonstrated safety and efficacy in several phase III RCTs and has recently received FDA approval for MC [95]. The remaining therapies are considered off-label. Notably, imiquimod has been found to be neither safe nor effective for treating MC [26]. Tretinoin, adapalene, and DPCP have only been investigated in small-scale studies for MC, although DPCP recently received FDA approval for use as a bulk drug substance in compounded topical medications [70]. DPCP is unique in its ability to clear lesions at locations other than the site of application [75]. Common adverse effects of all therapies include varying degrees of application site reactions. Additionally, topical retinoids often cause photosensitivity [49], and DPCP frequently leads to lymphadenopathy [63]. A wide range of less frequent adverse effects have also been reported for each therapy. In practice, DPCP is typically administered by clinicians, lacks standardized dosing protocols, and requires strict storage conditions [69, 71]. Conversely, patients and their caregivers can self-administer topical retinoids, imiquimod, and berdazimer sodium in standardized formu- lations, though berdazimer topical gel requires mixing two gels before application [95, 96], which may pose a challenge for adherence and compliance. Nevertheless, the convenience of home treatment with these therapies facilitates telemedicine follow-up, potentially expanding access to treatment [29].
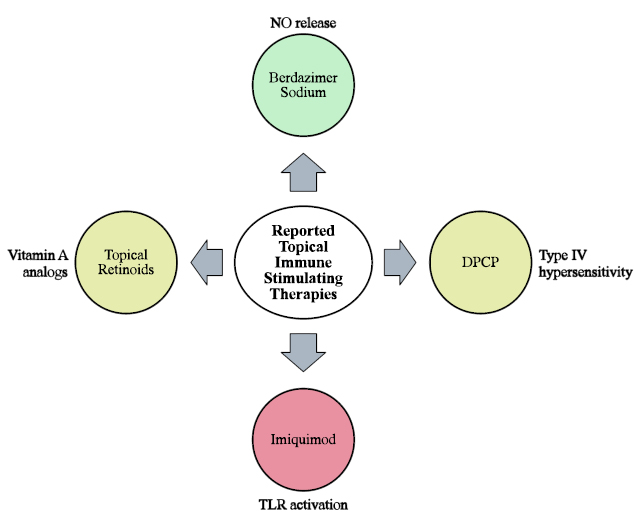
Summary of topical immune-stimulating therapies reported in the treatment of MC. Green indicates treatments currently FDA-approved for MC, yellow indicates treatments that require further investigation, and red indicates treatments no longer recommended for the treatment of MC.
Further research on topical immune stimulating therapies for MC is crucial given the current lack of universally effective treatments. Factors such as lesion quantity and distribution, patient age, and comorbidities influence the management of MC [8]. Unfortunately, only a limited number of studies have explored the efficacy of topical immune-stimulating therapies for genital MC or among immunocompromised individuals, who often present more severe manifestations of the condition [82]. Even FDA-approved berdazimer sodium has not been studied for treating MC in these populations [97], highlighting substantial research gaps. Advancing understanding of both MCV’s immune evasion strategies and the mechanism of action of topical immune-stimulating therapies could refine treatment options for diverse subgroups and facilitate the discovery of effective novel agents and combination therapies, leading to enhanced outcomes.
CONCLUSION
MC is an infectious skin condition that requires careful consideration of treatment options. Topical immune-stimulating therapies offer a minimally invasive approach, aiming to help the immune system reject MCV. Among these, imiquimod is no longer recommended for MC treatment, and further research is needed to assess the safety and efficacy of tretinoin, adapalene, and DPCP. Berdazimer sodium, however, recently received FDA approval for the treatment of MC following phase III trials. Despite these advancements, significant knowledge gaps remain regarding the mechanisms of these therapies and how they can be optimized for different MC presentations. Comprehensive long-term studies are needed to refine treatment strategies and ultimately alleviate the burden of MC on patients and caregivers.