All published articles of this journal are available on ScienceDirect.
Prolonged Mycobacterium Marinum Infection of the Hand, Wrist, and Forearm: A Case Report
Abstract
Introduction/Background
Mycobacterium marinum is a nontuberculous mycobacterium that can cause rare chronic skin and soft tissue infections in humans. Exposure is most common in individuals who work closely with marine animals or in marine environments, including aquarium caretakers and fishermen, and is typically acquired through a break in the skin or a fish bite. Diagnosis can be challenging due to its indolent nature and requires a high index of suspicion, including the elicitation of a water exposure history. As a result, it is often associated with delayed diagnoses, leading to chronic infections and related complications, such as arthritis, tenosynovitis, osteomyelitis, and bursitis.
Case Presentation
We present a case of a 59-year-old male who had previously worked as a fisherman and developed a chronic right upper extremity rash that was initially misdiagnosed as psoriasis, only later to be diagnosed as M. marinum infection 10 years after its initial onset. Laboratory confirmation was made through histopathology, tissue culture, and PCR.
Conclusion
This report highlights the epidemiology, clinical presentation, diagnostic difficulties, and complex management of Mycobacterium marinum infection.
1. INTRODUCTION
Mycobacterium marinum is a slow-growing, acid-fast, non-tuberculous mycobacterium (NTM) prevalent in aquatic environments [1-3]. It is a known pathogen in fish, causing necrotizing granulomas, and can rarely infect humans, leading to skin and soft tissue infections [1-3]. M. marinum is endemic in both fresh and saltwater, especially in stagnant or poorly disinfected bodies of water [4, 5]. In the United States, the estimated annual incidence is 0.27 per 100,000 population, accounting for up to 80% of NTM infections involving the hand [5].
M. marinum infections most commonly affect individuals who work closely with marine animals and habitats, such as aquarium caretakers and fishermen [2]. Transmission typically occurs through direct inoculation of broken skin with contaminated marine environment or direct contact with contaminated marine animals [3, 4]. Infections have also been reported following depilation, fish manicures, and trauma following bladed weapons [5]. M. marinum infection follows an indolent course, with an incubation period ranging from 2 to 8 weeks, but most commonly presenting within 3 to 4 weeks after exposure [1, 4, 5].
The clinical presentation is often non-specific and may include papules, nodules, plaques, pustules, or ulcers, typically localized to the extremities near the site of inoculation [4-8]. Cutaneous lesions may be solitary or multiple, and in up to one-third of cases, follow a sporotrichoid pattern if infection spreads along superficial lymphatic channels [4, 5, 7-9]. If left untreated, infection can progress to deeper tissues, resulting in tenosynovitis, bursitis, septic arthritis, and osteomyelitis [5, 6]. There are four recognized clinical classifications of Mycobacterium marinum infection based on severity and depth of tissue involvement: Type I – localized superficial cutaneous lesions; Type II – single or multiple granulomas; Type III – deep or more severe infections involving tissues such as joints, tendons, or bones, leading to arthritis, tenosynovitis, osteomyelitis, and/or bursitis; and Type IV – disseminated infection with involvement of the lungs or other systemic organs [5, 7].
We report a case of M. marinum infection diagnosed 10 years after the onset of a persistent rash, which had been previously misdiagnosed and treated as psoriasis. The patient presented with cutaneous lesions along with nail and hand deformities suggestive of onychodystrophy and tenosynovitis, consistent with stage III M. marinum infection. Unfortunately, treatment could not be initiated, as the patient was discharged before culture and PCR results were available, and he was ultimately lost to follow-up despite multiple contact attempts.
2. CASE PRESENTATION
The patient is a 59-year-old male with a history of polysubstance use who was admitted following a motor vehicle accident, during which a chronic rash on his right hand was incidentally discovered. The patient previously worked as a fisherman and developed the rash 10 years prior after sustaining abrasive injuries from fish tank glass. The skin had continued to thicken at the time and failed to heal. The patient reported pain associated with fissuring and bleeding of the thickened plaques and occasional pruritus. The condition was previously diagnosed and treated as psoriasis, failing several topical treatments.
Physical examination revealed a 10 cm dry plaque with thick scale, fissures, and crusts over an erythematous base that covered the dorsal aspect of the right hand, extending to his wrist, 5th digit, and proximal forearm, where a similar and reportedly newer 3 cm plaque had developed. The 5th digit was erythematous, edematous, with overlying thickened plaques and a thickened, yellow nail Figs. (1 and 2). Orthopedic exam showed hyperextension of the right 5th metacarpophalangeal (MCP) joint, and hyperflexion of the proximal interphalangeal (PIP) and distal interphalangeal (DIP) joints. Passive range of motion at the right 5th MCP was limited to 30 degrees extension, but flexion was within normal range at 90 degrees and without crepitus.
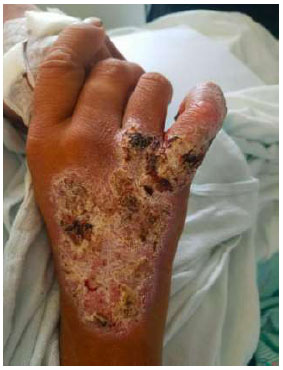
Dorsal view of the right hand. Dry plaque with thick scale, fissures, and crusts over an erythematous base covering the dorsal right hand, extending to his wrist and 5th digit.
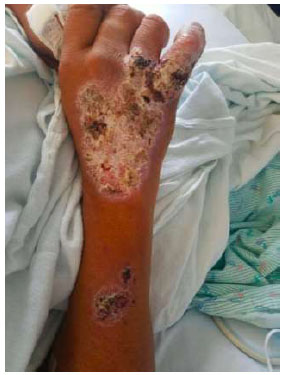
Dorsal view of right hand and forearm. Similar dry plaque with thick scale, fissuring, and crust is present over the right forearm, just superior to the main plaque; reportedly, it appeared 5 years after the initial injury.
Skin biopsies were obtained and sent for pathology. Bacterial, fungal, and mycobacterial cultures were also sent. Histopathology biopsy revealed mixed inflammation, without a predominant cell type Fig. (3). However, the fungal culture grew Aspergillus niger, which was suspected to be a contaminant. Three weeks later, tissue biopsy cultures yielded acid-fast bacilli, later speciated as Mycobacterium marinum Fig. (4). The PCR for the Mycobacterial tuberculosis complex was negative, and a follow-up PCR for non-tuberculosis mycobacteria was positive for M. marinum DNA. The patient’s clinical presentation of fixed flexion of the 5th digit was suggestive of underlying tenosynovitis due to M. marinum infection, indicating that this patient’s infection was at least stage II and possibly as severe as a stage III infection. Unfortunately, treatment could not be initiated because the patient was lost to follow-up, despite several attempts to contact them, including telephone calls and mailed letters Figs. (5-7).
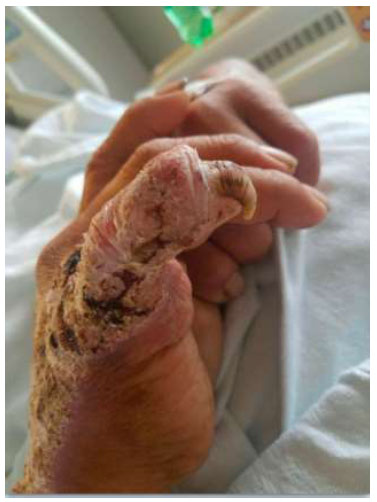
Medial view of the right hand. Dry plaque with thick scale, fissures, and crusts over an erythematous base covering the medial right hand, extending to the wrist and 5th digit. A thickened, yellow nail is noted at the 5th digit.
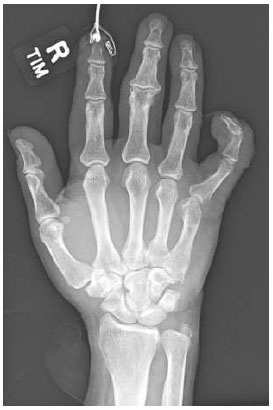
Frontal view of right hand x-ray. Hyperextension of the 5th MCP joint and subtle lucency at the neck of the 5th metacarpal. Soft tissue swelling and lacerations throughout the 5th finger.
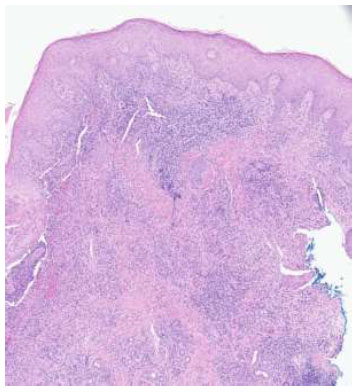
Punch biopsy at 4 times magnification. H&E-stained sections show epidermal acanthosis adjacent to ulceration with acute and chronic inflammatory infiltrate containing lymphocytes, plasma cells, numerous eosinophils, and focal granulomatous changes without distinct necrobiosis.
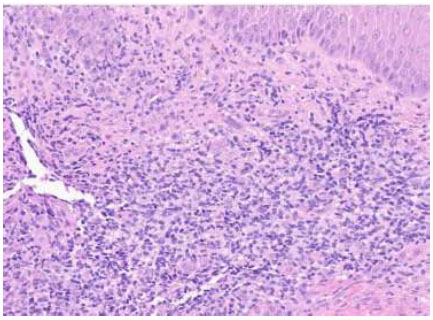
Punch biopsy at 20 times magnification. H&E-stained sections show epidermal acanthosis adjacent to ulceration with acute and chronic inflammatory infiltrate containing lymphocytes, plasma cells, numerous eosinophils, and focal granulomatous changes without distinct necrobiosis.
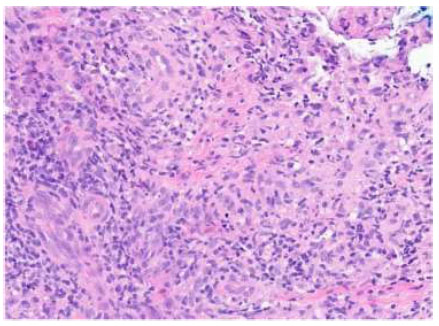
Punch biopsy at 40 times magnification. H&E-stained sections show epidermal acanthosis adjacent to ulceration with acute and chronic inflammatory infiltrate containing lymphocytes, plasma cells, numerous eosinophils, and focal granulomatous changes without distinct necrobiosis.
3. DISCUSSION
Mycobacterium marinum is a relatively rare cause of skin infection in humans, presenting unique diagnostic challenges due to its indolent course and resemblance to other chronic skin conditions, such as psoriasis. This often results in misdiagnosis and delayed recognition, as seen in this patient, who was initially diagnosed with psoriasis and ultimately identified as having M. marinum infection 10 years after symptom onset. Other possible misdiagnoses include tuberculosis (TB) verrucosa cutis, which has a similar appearance to M. marinum infection. Both conditions can present as verrucous plaques, most commonly on the hands. However, unlike TB verrucosa cutis, M. marinum infection initially presents as a pustule or nodule that later ulcerates and takes on a verrucous appearance. In this case, PCR testing ultimately confirmed M. marinum infection and excluded cutaneous TB. According to most published experiences, the time to diagnosis from first symptoms is approximately 15 weeks [5].
While M. marinum infection generally follows a benign progression, untreated infection can rarely progress to involve the nails and deeper structures, including tendons, bones, and joints, leading to complications such as onychodystrophy, osteomyelitis, tenosynovitis, bursitis, or arthritis. This patient exhibited clinical findings consistent with onychodystrophy and underlying tenosynovitis, indicative of a rare progression to Type III M. marinum infection.
Diagnosis relies heavily on clinical suspicion, supported by a detailed patient history including aquatic exposure, and confirmed by histopathology, tissue culture, and molecular testing [1, 6]. Histopathologic findings are diagnostic in approximately 50% of cases and typically reveal non-caseating granulomas with neutrophilic and lymphocytic infiltrates, macrophages, and multinucleated giant cells; early lesions may show collections of polymorphonuclear cells surrounded by histiocytes [1, 7]
The gold standard of diagnosis is the isolation of M. marinum from tissue culture; however, it has limitations [1, 7, 10]. M. marinum requires specific culture conditions and grows optimally on Lowenstein-Jensen medium at 30-32 degrees Celsius, with minimal to no growth observed at 37 degrees Celsius, which is the standard condition for typical pathogens [1, 4, 9]. Ziehl-Neelsen staining is often negative due to low bacterial burden [5]. Cultures must be maintained for 6-12 weeks, with growth often appearing within 2-5 weeks [1, 9, 10]. M. marinum is a photochromogen (Runyon group I), meaning colonies turn yellow when exposed to light (Fig. 8) [1, 6].
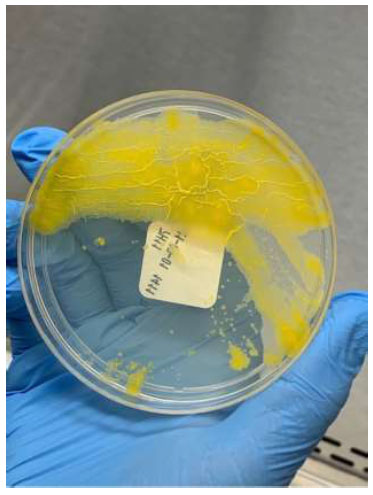
M. marinum on culture medium.
M. marinum is a photochromogenic pathogen, turning yellow on exposure to light.
However, culture yields are modest, with positivity rates ranging from 40% to 60%, further contributing to false negatives and diagnostic delays [1, 7, 10]. Adjunct molecular detection methods, such as 16S rRNA sequencing and matrix-assisted laser desorption/ionization-time of flight mass spectrometry (MALDI-TOF MS), are often used to aid in the rapid and accurate identification of M. marinum [1, 11-13]. Some species of Mycobacteria, such as M. marinum and M. ulcerans, remain difficult to distinguish due to their 99.6% genomic similarity [1, 11, 12]. In such cases, clinical history, along with M. marinum’s specific growth requirements and photochromogenicity, can aid in identification [11, 12]. In one small study, M. marinum and M. ulcerans were successfully differentiated using MALDI-TOF analysis of lipid profiles rather than protein signatures (Fig. 8) [12].
Due to the rarity of infection, there are no published randomized trials to guide optimal antimicrobial therapy [1, 4, 14]. Thus, treatment depends on clinical experience and preference rather than proven evidence [1, 5]. The 2007 joint guidelines of the American Thoracic Society (ATS) and the Infectious Diseases Society of America (IDSA) recommend dual antibiotic therapy for 1-2 months after symptom resolution, typically requiring 3-4 months of total treatment [1, 4, 5, 15]. M. marinum is intrinsically resistant to isoniazid and pyrazinamide [1, 5]. By standard susceptibility testing, it is susceptible to rifampin, rifabutin, ethambutol, macrolides (clarithromycin, azithromycin), sulfonamides, cotrimoxazole, linezolid, and imipenem [1, 4, 5, 14]. Treatment with fluoroquinolones (levofloxacin, ciprofloxacin) has also been reported [5]. Intermediate susceptibility has been reported for doxycycline, minocycline, and streptomycin [5]. Studies of minimal inhibitory concentrations (MIC) show that rifampin and rifabutin exhibit the lowest MIC (0.06-0.5 µg/mL). In contrast, other agents, including clarithromycin, tetracyclines, linezolid, imipenem, and cotrimoxazole, have MIC values near the breakpoint, indicating moderate activity [1, 5]. Routine susceptibility testing is not recommended and is reserved for cases of relapse or treatment failure [1, 5]. Notably, no acquired resistance has been reported in this organism to date [5].
Antibiotic regimens for Mycobacterium marinum infection vary, but commonly reported combinations include ethambutol with rifampin or clarithromycin, and rifampin with clarithromycin [5]. Frequently used dosages include: clarithromycin 500 mg orally twice daily, azithromycin (an effective alternative to clarithromycin) 250–500 mg orally once daily, ethambutol 15–25 mg/kg/day orally, and rifampin 600 mg orally once daily. Monotherapy with doxycycline (100 mg twice daily), minocycline (100 mg twice daily), and clarithromycin has been successful in treating mild, superficial infections, although treatment failures have also been reported [1, 5]. Deep or disseminated infections often require extended treatment courses lasting up to 18 months [1, 5]. Surgical intervention can be considered in cases with poor prognostic factors, including immunocompromised patients, refractory cases, and infections involving deeper tissue, although it remains controversial [1, 4, 5]. Novel treatments such as cryotherapy, x-ray therapy, and photodynamic therapy have also shown anecdotal success but lack sufficient evidence [1, 5].
The prognosis of treated M. marinum infection in immunocompetent patients is excellent, with treatment failure occurring in only 5.3-13% of cases [1]. Treatment failure appears to be associated with infection severity and deep tissue involvement rather than antimicrobial resistance [1, 5]. Delayed diagnosis can increase disease burden, necessitate longer treatment durations, and may result in greater complications and the need for surgery [1].
In this case, M. marinum was identified by tissue culture and PCR approximately 3 weeks after biopsy. Unfortunately, by that time, the patient had been discharged and was lost to follow-up, despite multiple attempts to contact them, precluding the initiation of therapy and assessment of clinical outcomes.
This case highlights several important considerations. First, it highlights the importance of timely and accurate diagnosis of M. marinum infection to prevent significant complications, including invasion of deeper structures and dissemination. In similar clinical scenarios, earlier diagnosis may be facilitated by obtaining a thorough patient history, with particular attention to both recent and remote environmental exposures. A high index of suspicion for M. marinum should be maintained in patients with a history of exposure to aquatic environments. Recognizing characteristic clinical features, such as a sporotrichoid pattern of spread, can aid in the early identification of the disease. Secondly, this case highlights the importance of maintaining a broad differential diagnosis and considering M. marinum in cases of chronic cutaneous lesions on the extremities that fail standard treatment, including topical steroids and standard antibiotic therapy. Lastly, it highlights the diagnostic challenges, including similarities in appearance to other skin conditions, prolonged incubation periods, and the limited sensitivity of cultures. In the appropriate clinical context, prompt and appropriate laboratory testing can significantly reduce delays and the risk of severe complications.
CONCLUSION
Individuals at increased risk of M. marinum infection should take appropriate precautions during related activities, including the use of protective equipment and avoiding marine exposure when the skin barrier is compromised. Clinicians should maintain a high index of suspicion for M. marinum when evaluating treatment-resistant cutaneous lesions, particularly in patients with a recent or remote history of exposure to aquatic environments. Timely and accurate diagnosis is important to prevent serious complications from prolonged infection, and to avoid inappropriate treatments that may be costly or harmful to the patient.
The indolent nature of M. marinum, combined with diagnostic challenges, lengthy culture incubation periods, and prolonged treatment requirements, underscores the importance of patient follow-up and retention strategies. Particular attention should be given to potential barriers to continuity of care, such as substance use and socioeconomic factors, to ensure effective treatment and prevent poor outcomes.
AUTHORS’ CONTRIBUTIONS
It is hereby acknowledged that all authors have accepted responsibility for the manuscript's content and consented to its submission. They have meticulously reviewed all results and unanimously approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| IDSA | = Infectious Diseases Society of America |
| MCP | = Metacarpophalangeal |
| ATS | = American Thoracic Society |


