All published articles of this journal are available on ScienceDirect.
Tissue Engineering in Cosmetics and Dermatology: A New Era of Innovation and Application
Abstract
The convergence of tissue engineering and the cosmetic industry marks a transformative axis in the development of advanced cosmetic products and therapies. The current study explores the recent landscape and future potential of applying tissue engineering techniques within the cosmetic industry. The study highlights key innovations, such as the development of lab-grown skin for product testing and personalized skin grafts for aesthetic enhancements, which not only promise to enhance product efficacy and safety but also offer sustainable and ethical alternatives to traditional methods reliant on animal testing. This study reviews scientific progress in biomaterials, scaffold design, and cellular manipulation that promote skin tissue regeneration and repair, wound healing, breast implants, and oral care, highlighting the capacity to tackle intricate aesthetic issues such as scarring, skin aging, and pigmentation disorders. The ethical, regulatory, and economic implications of integrating tissue engineering into cosmetics are also discussed, providing a comprehensive overview of the challenges and opportunities facing this burgeoning field. By advancing the capabilities of cosmetic applications, tissue engineering not only pioneers innovative solutions for personalized beauty care and dermatology but also sets a precedent for future interdisciplinary collaborations in cosmetic science.
1. INTRODUCTION
Tissue engineering and regeneration, a branch of biomedical engineering, involves the cultivation of different types of bodily tissues in vitro. The process involves utilizing cells, combined with natural or synthetic biomaterials that have been specifically engineered, and carefully adjusting biochemical and physicochemical parameters [1]. Establishing a conducive and controlled setting to encourage the growth and differentiation of cells is crucial for the success of cell-based tissue regeneration. The use of biomaterial technology plays a significant role in creating this cellular environment. One example is the exploration of drug delivery systems that incorporate bio-signaling agents and biomaterial scaffolds to amplify the potential for cell proliferation and differentiation, ultimately resulting in tissue regeneration. These scaffold and drug delivery technologies also drive forward stem cell biology and medical research while enabling the production of a surplus of high-quality cells for transplant therapy [2]. The field of cell research and therapy can greatly benefit from an innovative approach that involves genetic engineering to manipulate cellular functions [3, 4]. To highlight the crucial role of biomaterial technology in rapidly evolving research and therapeutic areas, this study provides several examples of tissue engineering applications that utilize cell scaffolds and drug delivery systems for growth factors and genes.
The creation of new, living tissue for medical purposes can be achieved through tissue engineering, which involves the use of cells placed on tissue scaffolds. However, it is important to note that this technique is not solely limited to applications involving cell and tissue scaffolds [5]. One of the goals of tissue engineering is the bio-production of autologous organs, tissues, vascularized to heal and replace complicated defects, organ scaffolds, and injectable biomaterials, designing fillers, and so on, in the cosmetics industry [6]. In this regard, biomaterials are used in creams, sunscreens [7], breast and tooth implants [8], as well as fillers [9]. They are also used in wound dressing in postoperative wounds and chronic wounds such as diabetic ulcers, which in addition to repairing the wound site, prevent scarring [10].
The term “cosmeceutical” was first introduced by RE Reed in 1962 [7]. Cosmetics consist of blends of chemical compounds derived from either natural or synthetic sources [11]. Cosmetics serve various purposes, including cleansing and protecting the body or skin, as well as altering appearance, perfuming, and/or addressing body odors, while also providing protection to the skin and maintaining its good condition. Various cosmetic products are used on different external parts of the body, including the epidermis, nails, hair, lips, and external genital organs, as well as on the mucous membranes of the oral cavity and teeth. However, it is important to note that these products are not intended to provide treatment for specific diseases [7]. Cosmetic formulations use different substances to perform various functions, including rheology modifiers, thickeners, foam stabilizers and destabilizers, emulsifiers, fixatives, conditioning agents, and film formers [1].
With growing animal welfare issues, it has become necessary to develop novel skin model substitutes in order to evaluate the effectiveness of pharmaceutical, skincare, and cosmetic goods using in vitro methods. It is crucial to possess tissue models that imitate natural skin in the dermo-cosmetology field to demonstrate the efficiency of bioactive ingredients or finished products effectively [6]. The field of cosmetology can greatly benefit from tissue engineering and regenerative medicine [12]. Tissue models that accurately reflect natural skin are required in the field of dermo-cosmetology in order to demonstrate the efficacy of bioactive ingredients or completed goods [13]. In order to address these new expectations in the cosmetics and skincare industries, skin tissue engineering is a crucial tool since it offers cutting-edge technologies that allow for the fabrication of intricate structures using living cells. Many skin conditions that affect a patient's overall appearance and self-esteem can be treated with tissue-designed replacements; in these cases, skin substitutes and cell therapies may have significant clinical implications. These alternatives are produced as biodegradable synthetic polymers . These biomaterials' porous structures allow for sufficient cell adherence to the matrix, while their hydrophilic qualities enable the delivery of nutrients and low-molecular solutes to the cells. Scaffold matrices find usage in multiple applications, including serving as a substitute for missing tissue, offering structural reinforcement, and carrying growth factors and/or cells that can develop into tissues upon transplantation into the body [14]. In this comprehensive review, we discuss tissue engineering applications and new findings in the cosmetic industry. In this review, we carried out a comprehensive review using PubMed and Scopus. Keywords and mesh terms related to “tissue engineering, biomaterials, cell-based therapy, regenerative medicine, aesthetic medicine, cosmetic dermatology, cosmetic procedures, dermatological research, and skincare” were used in several combinations. Moreover, additional relevant publications were identified through manual searching of reference lists. An experienced team of researchers selected and verified the studies.
2. APPLICATION OF TISSUE ENGINEERING IN COSMETICS
The appearance of skin is impacted by several factors, including age, gender, ethnicity, air contamination, nourishment, smoking, and sun exposure, and some people try to solve this problem by using many traditional cosmetic approaches such as skin’s creams, gel, lotions, lasers, etc [15]. Recently, tissue engineering has opened a new window in cosmetic industries, which provide many tools for cosmetic approaches Fig. (1). In this respect, various biomaterials used as fillers, ingredients in creams and lotions and biomaterials used in plastic surgery or cosmetic surgery are part of tissue engineering approaches.
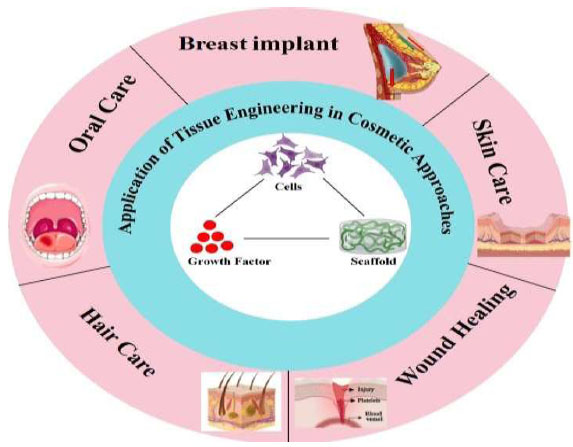
Schematic illustration for the summary of tissue engineering application in cosmetics and skin care.
Biomaterials, whether natural or synthetic polymers, function as structural supports for tissue regeneration or as carriers for drug delivery [16, 17]. They present diverse biopharmaceutical and physicochemical properties based on their natural, semi-synthetic, or synthetic composition and are utilized in skincare, dental care, nail care, and hair care. Both natural biomaterials and synthetic materials possess distinct pros and cons. Natural biomaterials provide significant biocompatibility, biomimicry, and even cost utility [16, 18]. However, low mechanical strength and high variability make them important. Synthetic biomaterials possess potential for modifications and regulated characteristics; yet, they exhibit limited biocompatibility [18]. The active compounds in cosmetics can be delivered effectively with the help of these materials, which play an important role in creating high-performance products with favorable attributes [8, 19]. Natural polymers are sought after for their biocompatibility, safety, and biodegradability, making them highly suitable for makeup, skincare, hair care, as well as stabilizers and modifiers [20]. Biomaterials are commonly used in the cosmetic industry for plastic and aesthetic surgeries. These biomaterials are categorized as plastic cosmetic biomaterials and can be further categorized into injectable biomaterials and prosthesis materials based on their specific properties [8]. Injectable biomaterials are predominantly employed for tissue healing, facial reconstruction, and deformity correction, while prosthesis biomaterials are commonly utilized for chin and rhinoplasty, breast augmentation, and temporal prostheses [21, 22].
Biomaterials have become an essential component of plastic surgery, a specialized field that involves the restoration, reconstruction, or modification of the human body [6]. Two major areas of plastic surgery are reconstructive and cosmetic surgery. While cosmetic procedures aim to enhance the appearance of a body part, reconstructive surgery focuses on the restoration or improvement of its function. Currently, plastic and cosmetic injections are widely used in reconstructive and aesthetic procedures, such as breast augmentation, rhinoplasty, and facelifts [8, 23]. In this regard, tissue engineering scaffolds are made to imitate the function of natural extracellular matrices (ECM), including collagen, gelatin (a hydrolyzed form of collagen), elastin, and hyaluronic acid [24]. Scaffolds can also be used to seed cells in vitro or to deliver growth factors and drugs. Scaffolds come in two types: solid and gel. Solid scaffolds must be surgically implanted, while gel scaffolds can be injected, depending on the type of deficiency in place [25]. Improving the way remedial growth components are delivered can result in a better and more effective restoration of skin tissues, both in terms of value and amount. This can enhance the efficiency of the process. Recent studies have shown that using a combination of growth factors and therapeutic agents can significantly improve the regeneration of damaged skin tissue compared to using only one growth factor [24]. To ensure the maximum effectiveness and stability of internally delivered growth factors, it is crucial to incorporate tissue-engineered biomaterials and related composites as implantable platforms [26]. The successful development and deployment of skin tissue engineering platforms could greatly enhance clinical skin regeneration. In addition to using available biomaterials for scaffold fabrication, researchers are also exploring the use of cell sheets and acellularized tissues for scaffolding and skin regeneration [27].
Researchers in the field of regenerative medicine are investigating the possibilities of utilizing three-dimensional (3D) printing technology for the production of customized scaffolds that can promote the regrowth of both firm and soft tissues [26]. This technology has the potential to assist with cosmetic procedures [28]. Building upon advancements in stem cell and tissue engineering research, 3D bioprinting holds the potential to generate bioactive tissues or organs in laboratory settings using growth factors and living cells as printing materials. This innovative manufacturing technology presents a viable option for leveraging biomaterials in plastic surgery and cosmetic applications, including maxillofacial and craniofacial reconstruction, nose and ear reconstruction, breast reconstruction [29], skin printing, and other areas. The development of bone replacements, personalized prostheses, and implants has great potential, this potential is especially noteworthy [8, 30]. For example, DeFazio et al. utilized a 3D printer to produce breast and nipple-areola prosthetics, which have demonstrated success in post-breast cancer surgery reconstruction, leading to a significant reduction in postoperative complications [31]. The process of “biological 3D manufacturing” involves replicating the construction and role of human tissues and organs while exhibiting certain physiological activities [26]. This process is conducted under specific conditions and is in line with the latest research advancements in regenerative medicine, tissue engineering, and stem cells [8].
One of the most appropriate options for use in different cosmetic methods is hydrogels. These gels are made up of networks of macromolecules that are cross-linked and have a high ability to absorb water. The swelling ability and mechanical strength are critical characteristics of hydrogel networks for cosmetic applications [19]. Cosmetic preparations often utilize hydrogels containing a range of natural biopolymers like gelatin, collagen, and hyaluronate. These innovative biopolymer-based hydrogels are being used to develop exciting new cosmetic products, including beauty masks designed to boost skin hydration, restore suppleness, and promote anti-aging benefits. By maintaining skin moisture, supporting skin health, delivering comfort, and even helping to prevent diaper rash, these hydrogels are revolutionizing the way we care for our skin [32]. Hydrogels that incorporate natural biopolymers such as gelatin, collagen, and hyaluronate are frequently employed in cosmetic preparations. These hydrogels, which are based on innovative biopolymers, are being utilized to create exciting new cosmetic goods, including beauty masks that help to enhance skin hydration, restore flexibility, and provide anti-aging benefits. By keeping the skin hydrated, promoting skin health, providing comfort, and even aiding in the prevention of diaper rash, these hydrogels are changing the way we care for our skin [19]. Hydrogels are a perfect choice for the encapsulation of proteins, peptides, fragrances, antioxidants, sun filters, moisturizers, perfumes, and anti-aging, tanning, and whitening agents in cosmetics. Biologically active substances in cosmetics are often vulnerable to environmental factors like temperature, pH, light, and oxidation. Encapsulation has been suggested as a means to improve stability and safeguarding cosmetic products against degradation, while also enabling greater control over the release of active agents in cosmetic products [33]. Chitin is one of the most common polysaccharides found in nature and can be utilized in various applications on the body, including the skin, hair, gums, and teeth. Chitosan hydrogels, when used topically, act as humectants, which are beauty products designed to increase the moisture levels in the outer layers of the skin [7]. The current usage of chitosan hydrogels in cosmetics is influenced by the capability of chitosan to form a film and fix hair. In cosmetic procedures, hydrogel can also be employed as a filler. Fillers can be categorized into nondegradable and biodegradable subtypes, with pure hyaluronic acid being a representative example of the degradable ones. Silicone, polymethylmethacrylate (PMMA), calcium hydroxyapatite (CaHA), and polyacrylamide gel (PAAG), among others, are popular synthetic fillers utilized in cosmetic-tissue engineering [34].
3. TISSUE ENGINEERING AND SKINCARE
One of the crucial focuses in tissue engineering is addressing acute and chronic wounds in skincare. Numerous chronic wounds can lead to amputations and even mortality, resulting in significant healthcare expenses. Moreover, the availability of healthy donor tissue for surgical procedures is often limited. Lowering the risk of immune rejection and infection is possible by using alternative tissue. At present, skin tissue engineering has emerged as a highly optimistic field, leveraging cutting-edge techniques like bio-inking, bio-fabrication, and bio-printing, alongside advances in DNA microarray, proteomics, and stem cells [26, 35]. This innovative approach holds immense potential for enhancing key cellular processes such as proliferation, survival, and differentiation, thereby facilitating the effective restoration and repair of damaged skin tissue. Tissue engineering in the dermo-cosmetic industry, especially in skincare products, holds tremendous potential. The cosmetics and skincare industry represents a significant and rapidly expanding global market, driven by the continuous evolution of hygiene and beauty products [36, 37]. Within these two industries, there are five main categories. These categories consist of hair care products, skincare products, fragrances, personal care products, and color cosmetics [37]. The cosmetics industry can be divided into skin enhancement and makeup subcategories. Skin-enhancing or skincare products, including bio-active formulations, moisturizing products, tanning creams, and bleaching products, exert prolonged effects on the skin, remaining in place for significant durations. In contrast, makeup products are usually applied briefly and then removed. Consequently, skin mimics are essential for determining the optimal concentration of specific compounds to meet industry standards while ensuring consumer safety and regulatory compliance. The regulatory compliance is the main sensitive and critical aspect of engineered skin and related therapeutics in product development and testing. Skincare products, with functions like moisturizing, refreshing, and cleansing, are available in various formulations such as creams, lotions, scrubs, exfoliates, and serums. Common skin cleansers like soaps contain anionic surfactants with higher pH levels, while synthetic detergents have lower alkaline pH levels [38, 39]. Maintaining the equilibrium between the stratum corneum barrier and hygiene protection is critical for these products [38]. Conducting experiments on skincare products is crucial to precisely assess the influence of the great charge density of the carboxyl head group, which enhances protein binding on the skin's surface [40]. “Smart” cleansers are imperative for differentiating between sebum and oil-soluble skin soils from lipophilic substances in the intercellular lipids, as surfactants cannot do so [41]. Tissue engineering, by creating skin models, can facilitate the development of these products by replicating the interface between cleansers and the skin. An artificial epidermal skin replica called EpiSkin® can simulate the way a cleanser interacts with the skin. The model can be classified into two types - penetration and stimulation, which allow creators to evaluate the effect of various external factors. To perform initial studies on permeation and toxicity, commercially available products such as EpiDerm®, SkinEthic®, VitroSkin, Epidermal Skin Test 1000®, and CreativeBioArray are utilized [42]. Furthermore, in response to mounting concerns about animal welfare, the development of alternative skin models for evaluating the efficiency of skincare, therapeutic, and cosmetic goods through in vitro techniques has gained importance [43]. Historically, skincare and cosmetics products have been assessed using animal models, with studies on skin erosion and burning dating back to the 1940s, evidenced by tests like the Draize rabbit skin irritation test. The expansion of alternative models, such as in vivo animal and ex vivo human skin attitudes, through investment has resulted in the creation of innovative experimental techniques [44]. Animal testing of final cosmetic products or components for skincare was prohibited by the European Union's 7th amendment to the Cosmetics Command in the early 2000s. Regardless of the presence of non-animal testing alternatives, this created a marketing restriction. Consequently, the industry was compelled to pursue alternatives and new methods as the new gold standard [45]. Human skin is considered the gold standard for evaluating and testing cosmetic and skincare products due to its potential to yield more precise and less variable results. Considerable efforts have been made to develop living skin equivalents capable of replicating some or all aspects of natural skin structure. This has been achieved by utilizing allogeneic skin cells that have matured in layers and have been cultured on scaffolds derived from extracellular matrix proteins. The patent for this innovative approach was filed in the 1990s, and its commercialization gained momentum due to the cosmetics industry's desire to provide viable replacements to animal testing [44]. Leading manufacturers, including L'Oreal, have utilized tissue-engineered skin as an in vitro alternative for human skin in their Episkin product line and other skincare products. Impressive advancements in skin tissue engineering particularly in the development of skin models, living tissue equivalents, and protocols for assessing skin attributes, have enabled in vitro experiments using skin tissue engineering to flourish [46]. Human skin constructs that are created through engineering are utilized in vitro for evaluating chromosomal impairment resulting from the application of agents on the skin's surface. Compromised skin tests are conducted to examine the penetration of chemicals through injured skin, while full-thickness skin counterparts serve as intricate skin models for research purposes [47], and skin models for studying LED light in acne therapy [48]. Tissue models that mimic the natural skin with high fidelity are one of the most important points in the field of dermocosmetology which proves the efficacy of bioactive principles or final products [13]. The first in vitro model of human skin was a living skin equivalent created through normal human keratinocytes (NHKs), which proliferated and differentiated on a de-epidermized dermis. For instance, to evaluate the potential of sunscreens for supporting the skin from UV-associated harm, one of the tissue engineering methods involved creating full-thickness skin models from fibroblast-populated collagen matrices (dermal equivalents) covered by stratified NHKs [49].
Skin tissue engineering and 3D bioprinting are the main tools to confront these recent applications in the skincare market, as they have developed innovative technologies that allow for the presentation of complex structures with living cells. Therefore, skin counterparts play a vital role in conducting in vitro experiments and assessing the efficacy of skincare products and helpful treatments. Not only do these engineered tissue models offer accurate testing methods for skincare formulations, but they also aid numerous investigation endeavors aimed at developing treatments for conditions like psoriasis and melanoma [43]. To ensure a precise presentation of human skin, skin models must possess specific characteristics. According to the tissue engineering outlook, for supporting cell culture, the existence of a three-dimensional and biodegradable matrix is required [50]. A new approach to skin models is proposed in this perspective, which has the potential for rejuvenation. The cells are cultured and start to grow, while the scaffold beneath them disintegrates. Engineered tissues are commonly created using various techniques and patterns, and are derived from different skin models that consist of both dermal and epidermal layers. Depending on the intended skincare application, these models can be physically and chemically selected [44]. Nevertheless, a multi-layered, complete, biomimetic and accurate skin equivalent is required in research and product development. Certain companies, such as StrataTest® and Epiderm FT®, tend to produce bi-layered constructs to model both the dermal and epidermal layers, while there are still several limitations and many future outlooks to further assay. Epidermal models, such as in vitro patterns including EpiDerm®, EpiSkin®, and SkinEthic® are currently available on the market [42, 51].
3.1. 3D Bioprinting
The production of engineered full-thickness skin models has recently been made possible through bioprinting, which provides a high-throughput solution for the same [52]. Traditional methods of tissue engineering used to replicate the complex layers of skin are often hindered by their laborious, expensive, and skill-intensive nature. These techniques also suffer from size limitations, which can pose a challenge when dealing with the extensive surface area of the skin. In contrast, bioprinting offers a more efficient solution, allowing for the creation of larger, more intricate skin features at a faster pace and with minimal sample manipulation [53]. Most investments focus on this prominent substitute for alternative tests in animal models, efficacy, and ameliorating product precision. Global skin care brands such as L’Oréal and Procter & Gamble (P&G) are currently developing and researching 3D bioprinted skin models. P&G has become a famous name for evaluating 3D bioprinting and positioning it as a very strong enterprise for dermo-cosmetic innovations. Singaporean researchers were asked to present an offer for £27.4 million in funds for 3D bioprinted skin in 2015. The purpose of the project was to test Procter&Gamble's skin products [43]. An efficient manner of attaining a qualified bioprinted model system for treating various structural and skin care situations is by utilizing combinations of fibroblasts, keratinocytes, melanocytes, and stem cells [6]. Skin equivalents generally include populations of allogeneic skin cells that can be developed in sheets and cultured on scaffolds derived from ECM proteins. Extrusion bioprinting, laser-assisted, and inkjet deposition are some of the principal techniques to achieve tissue-engineered skin for dermocosmetical and skin care purposes [54]. Bioprinting can be achieved through either laser-assisted or inkjet deposition techniques. In laser-assisted bioprinting, a donor layer that responds to laser excitation is applied to the target area. The process enables precise control over the deposition of biomaterials and cells, including stem cells. This level of control enables the creation of microstructures and precise spatiotemporal regulation, which are critical for studying processes such as tissue shrinkage and biomimicry. The extrusion-based technique is more supported by cells that permit a combination of biological molecules using mechanical power to produce and deposit a continuous cylindrical flow of bio-ink [44, 54]. The progress made in the development of 3D bioprinted skin for purposes beyond the cosmetic trade is not as significant as that of other methods for constructing simulated skin, which have been in use for many years.
4. DEVELOPMENT OF SKIN CARE PRODUCTS IN WOUND HEALING
Hyaluronic acid (HA), due to its diverse biological functions, has emerged as a crucial biological material for wound healing. HA-based functional wound dressings have been recognized for their role as a principal extracellular matrix (ECM) component [55]. Skincare products comprising HA sponge infused with growth factors and cosmetic ingredients serve as a reparative mechanism in the wound healing procedure by restoring the skin's original protective covering through reparative mechanisms in the individual layers. Chemical exfoliation using chemical agents has been extensively employed in aesthetic reconstructive surgery and dermatology, capitalizing on the skin's regenerative potential [56]. The process consists of the application of a chemical substance that prompts skin exfoliation, subsequently initiating the development of fresh skin, resulting in the revitalization of the skin. Successful management of degenerative wounds resulting from chemical exfoliations necessitates high-quality skincare products to facilitate wound healing. Epidermal Growth Factor (EGF) is effective in promoting wound healing by encouraging the growth of fibroblasts, keratinocytes, and vascular endothelial cells. This results in the development of granulation tissue and re-epithelialization. Combining EGF with VC has shown even greater potential in stimulating fibroblasts to release vascular endothelial growth factor (VEGF) and hepatocyte growth factor (HGF). Wound dressings made of freeze-dried spongy sheets composed of HA and poly (γ-glutamic acid) (PGA), which contain bioactive components like VC, glucosylceramide (GC), and EGF, can provide moisturizing effects in the epidermis. The effectiveness of these skincare products, composed of HA spongy sheets with bioactive components, was evaluated in animal experiments using nude mice. The results showed that a spongy sheet made of HA and PGA promotes wound healing, while the sheet containing GC, VC, and EGF significantly enhances the remedial outcome [57]. A new skincare item has been developed with HA and collagen (Col) sponges that have EGF and beauty components to aid in the healing of wounds. This commercially available skincare product contains HA, Col, VC, EGF, and is augmented with PGA, GC, and Arg as cosmetic components [58]. In vitro experiments using a wound external model were conducted to validate the effectiveness of EGF, the amount of VEGF, and HGF produced by fibroblasts. The findings indicated that the skincare solution containing EGF had a successful outcome in activating fibroblasts to produce a more significant quantity of VEGF, resulting in a 25-fold increase in HGF release as compared to the lack of EGF. These results indicate that the EGF-based skincare product significantly stimulates fibroblast and keratinocyte proliferation and effectively enhances HGF synthesis, suggesting its promise for damaged skin surfaces [57, 59].
5. ROLE OF TISSUE ENGINEERING IN HAIR CARE
Customers of hair care products are increasingly searching for sustainable, natural and science-backed options that have a significant impact on their purchasing choices. For hair care products to be truly effective, they must target damage and aging-related issues by rejuvenating, repairing, and stabilizing the hair. Additionally, these products should nourish and restore the hair's smoothness and softness, resulting in shiny and youthful-looking hair, while making routine washing and other topical treatments easier. Since hair is made up of 95% keratins, large proteins carry 18-23 different amino acids. Hair care products must be carefully crafted with their intended purpose in mind, keeping in mind the amino acid conformation, hydrogen bonds between chains, and the condition of the hair fibers [60]. Provisional hair deformation is achieved by breaking hydrogen bonds or ionic linkages, which can be useful for hair setting. On the other hand, to reach firm distortion, it is necessary to break cystine bonds and then reform them in a new location. Essential characteristics of hair care products include their ability to effectively cleanse hair, removing excess dirt and oil from the surface. For example, shampoos should selectively target the hair fibers and scalp, absorbing and activating the chosen components at the level of the cuticle scales. To repair and improve damaged hair structure, a comprehensive understanding of the amino acid sequence and the various bonds connecting them is essential. For this purpose, it seems that polymeric polysaccharides like chitin and its derivatives offer significant advantages. These compounds are made up of N-acetyl-2-amido-2-deoxy-D-glucose units that are arranged in crystalline microfibrils and surrounded by a protein matrix. This matrix is similar in composition to HA and is highly hydrated, which helps maintain a sleek and supple hair structure [60, 61].
Chitin and chitosan nano-fibrils have exhibited potential in cosmetics, packaging applications, and personal care due to their renewability, non-toxicity, environmental and biological compatibility, as well as their antioxidant and antimicrobial effectiveness [62]. These properties make them suitable for application in hair cosmetics and various other areas. The potential of Chitin nanofibril-nano-lignin (CN-LG) complexes in producing various tissues characterized by activities such as anti-dandruff, UV protection, hair repair, and antimicrobial properties has been explored [63, 64]. Advanced biodegradable tissues with immunomodulatory, antioxidant, antibacterial, and skin-repairing activities have been manufactured using complex nanoparticles [65]. These nanoparticles are further enhanced by the antimicrobial properties of encapsulated silver [66]. Advanced technologies have the capability to generate a range of specialized tissues that possess unique properties such as anti-dandruff and UV-screen safeguard, as well as hair repair and other vital functions. With continued research and development, these groundbreaking nanoparticle complexes could prove useful in hair products, as either dry nanostructures or as a suspension in cosmeceutical carriers [67]. Studies have shown that chitin can be broken down into its individual components, including acetyl-glucosamine, glucosamine, and glucose, while lignin can be transformed into polyphenolic compounds that can aid in repairing hair fibers and scalp cells. These findings suggest that CN, due to its micro/nano dimensions that resemble skin keratin fibrils, could have valuable applications in hair care by exhibiting skin anti-inflammatory and repairing properties [60, 68].
6. TISSUE ENGINEERING AND ORAL CARE
In reviewing the application of tissue engineering in the cosmetics industry, we emphasized the impact of this knowledge on maintaining and restoring the beauty of the oral area. Therefore, we reviewed this category with a holistic view and divided it in several sections, including tooth enamel, cleft lip and palate, oral mucosa, lips, and lip fillers (Fig. 2).
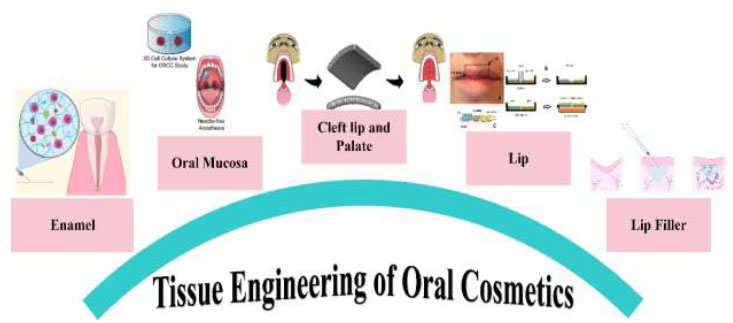
A schematic illustration for a summary of tissue engineering applications in oral cosmetics.
6.1. Enamel
Enamel is the body's toughest part, with its hardness determined by matrix proteins and the organized prism structure. Once teeth have matured, the body loses its ability to form enamel, making regeneration seem impossible. Nevertheless, innovative approaches such as material interaction, reassembly of matrix proteins, manipulation of cell-signaling pathways, and in-situ efforts such as laser irradiation have been utilized for enamel regeneration, protection, and mineralization [69, 70]. Amelogenin plays a crucial role as a key matrix protein and signaling molecule in enamel formation [70, 71]. Enamel expansion and formation depend heavily on the presence of thrombospondin 2 and matrix metalloproteinase-20 [72, 73]. The Ambn gene expression in dental epithelial cells can be enhanced by insulin growth factor 1, which has been demonstrated to induce enamel knots [74]. LRAP and mLRAP, which are modified forms of Leucine-Rich Amelogenin Peptide, and NAA, a non-amelogenin analog, all play a role in the restoration of prismatic enamel structures [75, 76]. Moreover, Amelotin and nanoengineered amelogenin in the presence of Ca2+ have been found to guide hydroxyapatite mineralization [77]. Finally, ameloblast-like cells and amelogenin proteins were observed after the transplantation of odontogenic collagen sponge and dental mesenchymal cells as tissue-engineered constructs into rats [78]. Human keratinocyte stem cells, upon administration of fibroblast growth factor 8 and Sonic hedgehog, exhibited remarkable efficacy in prompting the differentiation of enamel-secreting ameloblasts and expediting the production of enamel from adult stem cells [79].
From another point of view and in the field of material application, enamel-like calcium phosphate crystals were formed by applying fluorapatite/phosphoric acid (without H2O2) directly onto the human enamel thick layer [80]. In vitro, demineralized human enamel was effectively remineralized by a nanocomposite that included nanoparticles of amorphous calcium phosphate (NACP) [81]. Also, NACP exhibited a reduction in enamel demineralization around brackets in a caries model under acidic conditions to address orthodontic challenges [82]. β-pyrophosphate, which is doped with iron, has been found to possess the same level of rigidity as natural enamel. In comparison with dentine and a profitable composite, it is even harder [83]. Controlled nucleation-growth of an amorphous ZrO2 layer in situ was done. This layer shows strong adhesion to the enamel and resistance to bacterial adhesion and proliferation to restore enamel [84].
A toothpaste that includes amorphous calcium polyphosphate (polyP) microparticles and retinyl acetate (“a-polyP/RA-MP”) has been created to prevent dental issues. This toothpaste aims to seal cracks and fissures in enamel and dentin, fill cavities caused by tooth decay, and promote remineralization. By using this toothpaste, individuals can take a proactive approach to their oral health [85]. An innovative approach has been developed to fabricate a properly aligned enamel-like structure utilizing anodic aluminum oxide (AAO), a double-layered gel system, and polydopamine as a nucleating agent [86]. Laser utilization offers a promising avenue for enamel hardening and protection. Topical CO2 laser irradiation has been shown to enhance the mechanical properties of calcium-fluoride-like deposits, improve wear resistance, and increase fluoride uptake [87]. Furthermore, treatment with a laser and chelating agents has led to the formation of a fluorapatite crystal film on the enamel with similar prisms, showing potential for remineralization in saliva [88]. Lasers used for mineralization of bovine enamel have shown that calcium phosphate minerals doped with Fe3+ and femtosecond pulsed lasers encourage the formation of a new layer that is densely bonded to the fundamental natural enamel surface. This new layer exhibits higher resistance to acid than the natural enamel [89].
Although there are some successful attempts for enamel formation in vitro and in vivo in tissue engineering, applying that in a natural tooth is challenging, and it may be possible by the regeneration of entire teeth. Finally, in situ manipulation comprising material interaction or laser irradiation to protect or strengthen persisting enamel seems to be the most feasible way of enamel preservation and maintaining its aesthetic appeal. .
6.2. Oral Mucus
Naturally originated scaffolds are a promising option for the reconstruction of oral mucosa. In this regard, the combination of dog oral keratinocytes and AlloDermTM resulted in the formation of a multilayered epithelium that closely resembled normal oral mucosa [90]. The application of the complex did not enhance the recovery of palatal injuries in dogs [91]. However, the application of an oral mucosa equivalent produced outside the body (known as EVPOME), created by using AlloDermTM and a patient's oral keratinocytes, exhibited a better outcome in the healing of wounds [92]. A different research investigation that employed EVPOME discovered that patients who had their oral keratinocytes seeded on AlloDermTM did not encounter any negative responses or surgical complications. Moreover, the implantation of this complex resulted in the formation of augmented keratinized tissue around the teeth [93]. Additionally, the combination of Collagen-elastin matrix (MatriDerm®) with Gingival fibroblasts and keratinocytes demonstrated the ex-vivo growth of gingival tissue [94].
The use of the amniotic membrane in tissue engineering has become increasingly popular due to its promising potential (6). Oral keratinocytes cultured on amniotic membrane have demonstrated reassembly into multilayer epithelial cells containing desmosomes and hemidesmosomes similar to those in normal mucosa [95]. Moreover, human oral keratinocytes seeded on Hyper-dry amniotic membrane (HD-AM) within a serum-free culture system displayed a structure resembling native gingival mucosa and exhibited healing and durability in athymic mice [96]. Patients suffering from osteoradionecrosis start healing by transplantation of keratinocytes cultured on amniotic membrane and poly (L-lactic acid) to cover intro-oral fistulas and bone loss. This treatment resulted in successful healing for most of the patients [97]. It was possible to culture oral keratinocytes on the acellular porcine dermal medium, which resulted in the successful formation of a multilayered oral epithelium [98]. Researchers have developed a complete autologous oral mucosa equivalent (CAOME) using cells extracted directly from patients and a fibrin glue scaffold. The resulting monolayer epithelium closely resembled the basal layer of the oral mucosa. The researchers confirmed its regenerative potential through maturation in athymic mice [99, 100]. Notably, an autologous full-thickness tissue-engineered fibrin-based oral mucosa led to tissue regeneration in a pediatric patient with hemifacial microsomia and congenital ankyloglossia [101]. Additionally, combining fibrin-agarose biomaterials with fibroblasts and keratinocytes culture produced an artificial human oral mucosa model, while introducing various types of mesenchymal stem cells (MSCs) led to blood vessel formation following endothelial differentiation in the presence of human oral mucosa fibroblasts and fibrin-agarose support in athymic mice [102, 103]. Mice exhibited no immune rejection and minimal scarring when tissue-engineered oral mucosa-like structures with rabbit ACVM-0.25% HLC-I scaffold were used [104]. Among the regenerative-based approaches to treat maxillofacial deformities, It must be noted that stem cells, especially mesenchymal stem cells, have a significant impact [105].
Additionally, oral mucosa equivalent cultured from cryopreserved oral keratinocytes of the lip mucosa after four to six months of cryopreservation showed similarities to native palate mucosa tissue [106]. The dermal regeneration template (DRT) was found to be the best scaffold for tissue-engineered oral mucosa equivalents (OME), while an interpenetrating polymer network of poly-vinylpyrrolidone and poly (acrylic acid) showed increased porosity and metabolic activity when in interaction with oral mucosa cells compared to PAAc alone [107, 108]. Achieving adhesion at the hard/soft tissue interface is possible through the incorporation of NH2 and o-quinones functional groups, which promote tissue integration and cell ingrowth. In a similar fashion, researchers have created a composite osteo-mucosal system that merges soft and hard tissues using a scaffold made of hydroxyapatite/tri-calcium phosphate and collagen gel [109, 110]. Additionally, a fish scale collagen-derived scaffold imitating the dermal-epidermal connection of the oral mucosa has been fabricated using semiconductor process and soft lithography technologies [111]. These approaches demonstrate the potential of using naturally occurring scaffolds combined with oral mucosal cells to regenerate and heal oral mucosal defects through tissue-engineered alternatives, representing successful advancements in restoring oral aesthetics.
6.3. Cleft Palate and Lip
The approaches to tissue engineering and regeneration for cleft palate and lip share many similarities with the spatial application of natural origin scaffolds in oral mucosa reconstruction. For instance, Integra® Collagen-Glycosaminoglycan matrix has been implanted into full-thickness wounds on the palatal mucoperiosteum in dogs, leading to faster closure without the silicon layer, and with reduced parallel collagen fibers and myofibroblasts [112]. Additionally, a cell-free structure, resorbable poly (1,8-octamethylene-citrate) (POC) combined with decellularized human amnion membrane (DAM-POC), has demonstrated repair capabilities in large palate defects in rats [113]. Furthermore, decellularized porcine mucoperiosteum, seeded by human bone marrow-derived mesenchymal stem cells (hBM-MSCs), has been proposed as a suitable allogenic transplant for cleft lip and craniofacial reconstruction. These findings highlight the potential of naturally occurring scaffolds in similar applications for cleft palate and lip tissue engineering and regeneration, as well as oral mucosa reconstruction [114]. Cultured autogenous osteoblasts on demineralized bovine collagen matrix (Osteovit®), compared with spongious iliac bone as a control, were grafted in children with cleft lips and palates, demonstrating increased ossification in the tissue-engineered bone without any drawbacks compared to the gold standard [115]. In another study, submucoperiosteal implantation of cross-linked type I collagen scaffolds in the palates of rats directed the formation of new collagen. It elicited a transient inflammatory response, highlighting the need to inhibit myofibroblast differentiation [116]. Moreover, more efficient ossification was observed in undifferentiated MSCs with bovine hydroxyapatite/collagen (BHA) compared to osteogenically differentiated MSCs with BHA. A study involving a population of 10 patients comparing tissue-engineered constructs (collagen/osteogenically differentiated BM-MSCs) with anterior iliac crest grafting showed similar bone volume yields, indicating sufficiency for unilateral secondary alveolar cleft reconstruction [117, 118]. In a rabbit palate defect model, a scaffold made of fibrin-agarose and seeded with mesenchymal stem cells derived from adipose tissue, which had been previously transdifferentiated from osteoblasts, successfully produced bone tissue comparable to natural bone [119]. Using rabbit cells and hydrogels, scientists created a successful multilayered palate substitute that integrated into the host tissue. Although complete differentiation was not achieved, tissue maturation and differentiation were deemed adequate [120]. In goats, a composite scaffold containing hydroxyapatite/chitosan/gelatin, human umbilical cord-derived mesenchymal stem cells, and bone morphogenetic protein-2 effectively induced bone growth in critical size alveolar defects, replicating the regenerative ability of iliac crest alveolar bone implants [121]. In the context of regenerating the bony part of the palate, the composition of scaffolds varies. Specifically, in an osteogenic medium, adipose-derived stem cells were grafted with a hydroxyapatite/beta-tricalcium phosphate scaffold in a canine alveolar cleft model. Although the study indicated that autografting resulted in significantly greater bone formation, the tissue-engineered construct could potentially serve as an alternative [122]. In a different approach, rabbits with alveolar cleft defects were treated by grafting composite xenogenic dentin with beta-tricalcium phosphate. The results showed higher bone capacity element and residual graft volume compared to using beta-tricalcium phosphate alone [123]. In a rat alveolar cleft model, 3D printing of biodegradable calcium phosphate with and without rat mesenchymal stromal cells (rMSC) revealed that pore geometry significantly influenced bone formation, with a 60° strand rotation showing better results than a 30° strand rotation, and interestingly, the presence of rMSC did not improve bone formation [124]. Furthermore, a clinical trial suggested the potential efficacy of tissue-engineered cartilage using a poly-L-lactide scaffold in improving cleft lip-nose deformity [125]. Lastly, 3D-printed polycaprolactone scaffolds seeded with bone marrow-derived mesenchymal stem cells were proposed as a possible alternative for alveolar cleft reconstruction [126]. In a comparative study involving 24 cases, various grafting techniques for treating alveolar cleft defects were examined, including A) autogenous iliac crest bone, B) nano calcium hydroxyapatite with a collagen membrane, and C) bone marrow stem cell extract and PRP growth factors. The study concluded that nano calcium hydroxyapatite and bone marrow stem cell extract were reliable alternatives to autogenous iliac crest for bone grafting [127]. In a different study, orthodontic expansion in the graft area of tissue-engineered bone in a canine alveolar cleft model was found to reduce bone resorption, accelerate new bone formation, and enhance mineralization compared to the untreated and autogenous iliac bone [128]. Similar to oral mucosa, natural origin scaffolds have made significant contributions to cleft palate/lip regeneration. However, given the presence of hard tissue in this area, the use of mineral and polymeric materials is crucial. While autogenous iliac crest autograft remains the gold standard for cleft palate/lip reconstruction, numerous trials have demonstrated the effectiveness of tissue-engineered alternatives.
6.4. Lips
As the most visible part of the mouth, the lips often become the focus of beautification efforts, which may involve both simple and complex procedures, such as the use of fillers. Human skin and oral keratinocytes were seeded on AlloDermTM to reconstruct a continuous layer of the lip, comprising the epidermal skin, oral mucosa, and vermillion resembling full-thickness human lip skin equivalents. Additionally, a 3D tissue-engineered mucocutaneous structure encompassing distinct areas—skin, vermillion, and oral—was created using AlloDermTM, primary human skin keratinocytes, and oral mucosal epithelial cells [129, 130]. Furthermore, postmortem facial grafts, such as lips, were obtained from fresh human cadavers by decellularization with detergent and polar solvent. This process preserved an extracellular matrix suitable for cell growth, an intact vascular network, and the original morphology. This innovative approach could represent a significant advancement in allotransplantation [131].
6.5. Lip Filer
Patients who received collagen filler (Dermicol) treatment in areas such as nasolabial folds, lips, corners of the mouth, vermillion border, marionette lines, or a mixture of these, reported aesthetic satisfaction and resumed daily activities within a short time frame (2 to 7 days) [132]. Patients who received medical-grade liquid injectable silicone LS by the microdroplet technique for lip augmentation showed high satisfaction and minimal complications such as ecchymoses, haematoma, and small palpable nodules during 3 to 7 years of follow-up [133]. Polydioxanone (PDO) is biocompatible and naturally biodegradable with minimum inflammation. Stent-shaped multi-polydioxanone (SSMP) scaffolds were reported as another probable safe treatment for upper lip wrinkles after 12 weeks of follow-up [134]. Polymethylmethacrylate, a long-lasting cosmetic filler commonly used for wrinkle correction, has been associated with rare complications in facial and lip augmentation cases [135]. The palmaris longus tendon graft was examined in 38 patients for cosmetic and reconstructive purposes, proving to be a reliable filler for upper lip augmentation [136]. Agarose gel, evaluated in 68 cases over three years, was found to be a predictable and dependable filler for lip augmentation [137]. In comparison with control, Hyaluronic acid Restylane Kysse (HARK) was effective for lip augmentation and upper perioral wrinkle correction, supporting improvements in lip texture, color, and enhancement of the lip and perioral region [138, 139]. On the other hand, lipogranulomas were reported following the injection of unregistered substances for lip augmentation by inexperienced practitioners [140]. Fillers can stimulate collagen production and help restore lost tissue volume to some extent. When approved fillers are used under expert supervision, they generally exhibit good biocompatibility and can lead to satisfying outcomes for both patients and physicians. In summary, products such as commercial dermal grafts, biological scaffolds like collagen and hyaluronic acid, and materials for ossification play a significant role in enhancing the oral area through a tissue engineering approach.
7. TISSUE ENGINEERING AND BREAST IMPLANTS
Tissue engineering offers the possibility of producing stable and biocompatible implantable tissue, which may have a positive impact on breast aesthetics, in particular [141]. Several underlying factors are involved in cellular therapy and tissue engineering in breast-related manipulations. Despite aesthetic considerations, invasive procedures like mastectomy make regenerative medicine techniques essential for breast regeneration. For instance, nearly 2 million women worldwide are diagnosed with breast cancer each year [142], and roughly 40% of them will require a mastectomy to effectively manage their disease [143]. However, common breast reconstruction techniques have some disadvantages, such as infection, capsular contracture, rupture, the need for additional surgery in implant-based reconstructions, and complications at the donor site in autologous reconstructions [144]. Hence, scientific investigation has recently shifted towards adipose tissue engineering to overcome the mentioned disadvantages. While some defined scaffolds can provide suitable biochemical and biomechanical signals for adipogenesis, several scaffold designs and biomaterials have been employed to develop a diverse range of restorative strategies. Among these are hydrogels, which have been extensively studied for their potential to regenerate adipose tissue, since they are capable of faithfully mimicking the extracellular matrix, which is extremely useful [144]. In addition, hydrogels are highly biocompatible and biodegradable, making them ideal candidates for tissue engineering strategies in breast aesthetics.
Furthermore, in parallel to scaffold design, research has been conducted to develop ideal biomaterials for breast reconstruction. Biomaterials' progress has enabled us to utilize increasingly advanced materials in tissue engineering. One of the most beneficial candidates in this regard is collagen proteins, which are considered to be one of the most beneficial. In spite of this, enhancing the properties of collagen proteins is of great necessity due to the importance of breast aesthetics. As an example, collagen oligomers retain their ability to self-assemble, which is a characteristic of fibrillary collagen proteins [145, 146]. In addition, when oligomer solutions are neutralized to match physiological conditions, they may be easily used to fill intricate shapes and structures. In an organism, these scaffolds remain intact, undergoing a gradual metabolic cycle and restructuring. They are resistant to proteolytic enzyme breakdown and do not cause active breakdown or foreign body response. Therefore, prototype collagen oligomer formulations for breast reconstruction are being developed in order to address these needs [147].
Alternatively, scaffolds can be introduced into the breast and then seeded, instead of being cultured in a laboratory setting, which is currently the conventional method for creating tissue-engineered products [148]. In this method, the host tissue serves as a bioreactor, in which endothelial cells penetrate the scaffold to stimulate the formation of new blood vessels prior to seeding in vivo [148]. However, mixed lipoaspirate may have an increased proliferation capacity than isolated adipose stem cells, which adheres to the tissue engineering concept of angiogenesis before adipogenesis. Furthermore, recent research has underscored the potential of adipose-derived stem cells as a therapeutic agent for keloids and wound healing by reducing the fibrotic effects of TGF-β on keloid-derived fibroblasts [149].
Additionally, nipple‐areola complex (NAC) regeneration is a common intervention performed alongside breast reconstruction. To reconstruct the nipple, local skin flaps have traditionally been employed; however, more recent approaches have utilized implanted materials. Several studies have been conducted to evaluate the use of artificial structures in the reconstruction of augmented-flap NACs [148]. Despite being highly effective in maintaining nipple projection, most of the materials were correlated with adverse effects. For recreating permanent nipple mound projections, alloplastic materials such as tissue-engineered nipples, silicone rubber endoprostheses, custom-made nipple endoprostheses, and polyurethane prostheses are applied [150, 151]. While these procedures are associated with an increased risk of necrosis and cosmetic loss, more research is needed in this area.
8. FDA-APPROVED BIOMATERIALS FOR COSMETIC APPLICATION
Biomaterials are engineered substances designed to interact harmoniously with biological tissues within the human body. These materials can be synthetically produced or sourced from natural origins [24]. Over the past five decades, significant advancements have been made in the development and utilization of biomaterials across a wide range of therapeutic applications [152]. Projections indicate a consistent growth trajectory for the global biomaterials market, with an anticipated annual growth rate of 15.97%. By the year 2027, this market is forecasted to achieve a substantial value of USD 348.4 billion [153]. The term “cosmetic” pertains to any substance or formulation intended for external application on various parts of the human body, including the mucous membranes surrounding the oral cavity and teeth. Regulatory organs such as the European Union (EU) and the United States Food and Drug Administration (FDA) oversee the safety and efficacy of cosmetics. They establish and update the guidelines and regulations for the manipulation, manufacture, packaging, and distribution of cosmetic products to ensure consumer protection. Bioprinted products are classified as medical devices and biologics, depending on their intended use. Bioprinting-based products must follow the FDA's regulatory pathways, which include premarket notification, premarket approval (PMA), and/or investigational new drug (IND) application. Cell- and tissue-based therapies are classified under the FDA's regulations for vaccines, blood and biologics [154]. Beyond concealing natural odors, cosmetics play diverse roles such as enhancing physical appearance, promoting skin and hair health, providing fragrance, and offering protective benefits [19].
Before being sold in the United States, cosmetic ingredients and products are not required to receive FDA approval. Color additives are the lone exception, and they must be certified for their intended usage (apart from the coloring components used in coal-tar hair colors). Businesses and individuals who sell cosmetics are required by law to ensure the security of their products. There has been a need for reliable evidence showing that a cosmetic offered on the market is dangerous when used by customers as directed by the labeling or in the customary or anticipated way [155].
Tables 1 and 2 summarize some of the FDA-approved biomaterials and list some of the commercial products made from natural and synthetic biomaterials, respectively.
| Products | Component | Application | Reference | License |
|---|---|---|---|---|
| Alginic Acid | Alginate | Protective colloid agent, Viscosifying, Moisturizing Emulsifier Colloids, Gelling, Chelating Immunostimulating, |
[193] | FDA approved |
| Eastman™ Cellulose Acetate Propionate | Cellulose | Nail care Formulating nail lacquer topcoats |
[194] | FDA approved |
| Gelatin | Gelatin | Improves skin health Causes skin firmness |
[153] | FDA approved |
| Collagen | Collagen | Boosts skin hydration Reduces wrinkles Improves skin elasticity |
[160] | FDA approved |
| Restylane® | Hyaluronic acid | Aesthetic plastic surgery Soft-tissue fillers lead to smoother skin |
[195] | FDA approved |
| Dermaroller® | Chitosan | Skin & hair care Oral hygiene products |
[196] | FDA approved |
| Shandong Freda Biotechnology-Xanthan gum | Extracellular bacterial polysaccharide | Skin care formulations Lotions Oral care products. |
[197] | FDA approved |
| Products | Component | Application | Reference | License |
|---|---|---|---|---|
| Penuma® | Silicone | Penile silicone sleeve implant first developed in 2004, is the first FDA-approved penile implant for cosmetic enhancement. |
[198] | FDA approved |
| Gore-Tex® | ePTFE (polytetrafluoroethylene) | Facial implant material | [199] | FDA approved |
| SAM-KLG 95 | Potassium Lauroyl Glutamate | Formulating shampoos and Facial cleansers Shower gels Baby products. |
[200] | FDA approved |
| Botox® | PMMA Poly(methyl methacrylate) | Botox and dermal fillers | [201] | FDA approved |
| Gotalene® 120 colored 23 | High-density polyethylene | Repair of cheeks Orbital arch Orbital floor, upper and lower jaws, cheekbones, temporal, and ears |
[202] | FDA approved |
| Asensa® NSC 22 | PLA Poly(lactic acid) |
Surgical absorbable sutures, Bone fixation plates Exfoliating face gels and creams Body scrub Shower gels Creams Butters Hand soap cleansers Exfoliating foot care, Hair care |
[203] | FDA approved |
| Ganex® V216 | Poly Lactic-co-Glycolic Acid (PLGA) | Pigment dispersant or suspending agent. |
[204] | FDA approved |
| PromaCare® SCP | Hydroxyapatite | Repair of bone defects such as oral and maxillofacial regions, Substitutes for teeth, Blusher, Loose powder Toner Tone-up cream formulations |
[205] | FDA approved |
| 45S5 Bioglass® | Bioglass materials | Bone repair and regeneration | [206] | FDA approved |
8.1. Naturals Biomaterials
Gelatin and collagen, which are natural biomaterials, have demonstrated promising results in cosmetic applications. Additionally, natural biomaterials that resemble the extracellular matrix, such as chitosan, cellulose, hyaluronic acid, and alginate, are highly biocompatible with human tissues [156, 157]. Due to this characteristic, these materials are frequently used in the creation of industrial cosmetics as well as in vitro and in vivo testing for implanted medical items [156, 158].
Animal creatures contain the structural protein collagen, which offers the basic structural support. It is often derived from fish and mammalian skin. Collagen has become the subject of significant academic research and has garnered attention from the beauty industry due to its fascinating properties, such as its ability to naturally hydrate and moisturize the skin as a humectant [159].
Multiple clinical trials on bovine collagen over a 6-year period were conducted to reduce age-related wrinkles. In 1981, the FDA gave it its first cosmetic approval. Prior to injecting into the face, pre-testing for allergies must be done because sensitivity to such substances is conceivable [160]. Due to its moisturizing, renewing, and film-forming qualities, collagen is one of the major ingredients in cosmetic compositions. The skin's proper water content is maintained throughout the day thanks to its excellent capacity to bind water. The skin is hydrated and becomes softer. It is a skin-like substance made from animal sources. It provides a distinctive skin-feel and acts as a water reservoir on the skin, both of which contribute to the hydration of the skin. Additionally, collagen is a component of skin care treatments. The most essential structural component of the skin is collagen. The FDA has approved dermal fillers made from both cow collagen and bioengineered human collagen [161].
Hyaluronic acid (HA) was developed as a biocompatible substitute for collagen, offering longer-lasting results. This extracellular glycosaminoglycan is highly hydrophilic and has gained FDA approval for cosmetic use since 2003, leading to the development of new filler materials between 2003 and 2007. HA is a vital component of the extracellular matrix, present in tissues like the eyes, synovium, and skin. De Maio has explored the efficacy of HA fillers in altering muscle function, proposing that these fillers could impact muscle contractions mechanically by either facilitating or inhibiting them [162].
Alginates, a type of natural polysaccharide, are derived from the cell walls of numerous species of brown algae. This biopolymer consists of a linear structure composed of [1-4]-linked b-D-mannuronic acid (M) and a-L-guluronic acid (G) residues, which are arranged in varying proportions and sequences. Apart from the food and pharmaceutical sectors, the demand for cosmetics has been on the rise in South America and Asia. In 2014 alone, the global beauty product market attracted investments totaling around 250 million euros, with increasing cosmetic consumption observed in countries like China (32.6 million), Brazil (32.6 million), and India (8 million) [163].
According to reports, nitrocellulose serves as a film maker, a nonsurfactant dispersing agent, and a dispersing agent in nail polish formulas. It is also used frequently in cosmetics [164]. In its Voluntary Cosmetic Registration Program, the FDA solicits data from manufacturers regarding the use of specific substances in several categories of cosmetic products (VCRP). Nitrocellulose is reportedly utilized in 516 nail product formulations and 1 “other” makeup composition, according to VCRP data released from the FDA in 2013 [165]. In 67 of the 79 basecoat and undercoat formulations, and in 410 of the 489 nail polish and enamel formulations that were submitted to the VCRP, nitrocellulose was used [166].
Chitosan is another polysaccharide that is linear and made up of 1-4 linked 2-acetamido-2-deoxy-β-D-glucopyranose. It is a N-acetyl-D-glucosamine and D-glucosamine copolymer. Chemically speaking, chitosan is a chitin derivative that is produced by partially deacetylating chitin [167, 168]. Crustaceans like crabs, lobsters, shrimp, and krill are used to remove chitin [169, 170]. Chitosan is the name given to a substance (chitin) if it contains less than 50% acetyl glucosamine [171]. Because chitosan can form films and is biodegradable and biocompatible, it can be used to make microneedles (MNs), which are ideal for topical and transdermal medication delivery. Chitosan can be modified to have tunable characteristics and functionalities thanks to the presence of amine and hydroxyl functional groups. In this regard, chitosan is the preferred material for making MNs since it can be customized to the appropriate strength and functions and does not trigger an immune response in the body. As a result, numerous researchers have tried to employ chitosan as a drug delivery system for hormones, peptides, and hydrophilic medications. The FDA published “Regulatory Considerations for Microneedling Products” in 2020. The development of MNs will have prospects in the future thanks to this official advice. The guidance helps ensure the safety and efficacy of MN products on the consumer market. Currently, there are MN products on the market, with the majority being used for skin care and cosmetics, including Dermaroller®, MicroHyala®, LiteClear®, MS-4, MF-8, C-8, and CIT-8 [172, 173].
8.2. Synthetic Biomaterials
Synthetic biomaterials are formed from one or more types of monomers that are covalently bonded to one another, creating macromolecules. They comprise a wide category of compounds, with variations mostly due to the kind and quantity of monomer units [174, 175]. The synthetic polymers are appealing as an excipient in the formulation of cosmetics because they may be customized for particular applications. They may be made in huge quantities with consistency, are frequently less expensive than natural polymers, and have a lengthy shelf life. The most commonly utilized synthetic polymers in cosmetics include acrylic acid-derived polymers, silicones, polyacrylamides, as well as homopolymers and copolymers based on alkylene oxides [176].
Plastic surgery encompasses a comprehensive spectrum that includes maxillofacial procedures, liposuction, skin flaps and grafts, breast surgeries, body contouring, and facial aesthetics [8, 177]. This multifaceted discipline relies heavily upon biomaterials due to their exceptional biocompatibility and biodegradable properties, which ensure the utmost biological safety required by these surgical interventions [177].
Although compared to synthetic polymers, materials generated from nature are better able to mimic real ECM, improve cell adherence, and control cell proliferation, they are advantageous for cellular activities, but synthetic polymers provide stronger mechanical strength, greater processability, and predictable breakdown rates [178, 179]. These characteristics render them well-suited for incorporation into scaffolds used in 3D printing. Poly(D, L-lactic-co-glycolic acid) (PLGA) and poly(e-caprolactone) (PCL) are the two synthetic polymers that are most frequently utilized [8].
During the mid-20th century, silicone emerged as a prominent material in the realm of healthcare due to its exceptional characteristics. Silicone fulfills the requirements for high-quality medical polymers owing to its advantages, which include thermal stability, low temperature tolerance, non-toxic nature, resistance against biological degradation, minimal interaction with living tissue, physiological neutrality, and favorable physical and mechanical attributes [180]. Consequently, its adoption within the medical sector has expanded significantly.
Medical silicone materials are being used on many different regions of the human body, including the nose and the skull, for aesthetic surgery and internal organ repair. Silicone can be used as a prosthesis to restore the body's surface in addition to being implanted within the body. Due to its moderate strength, soft texture, high biocompatibility, and excellent operability, silicone has gradually become the preferred material for creating facial prostheses [181, 182]. It is used to treat external ear deformities and maxillofacial defects caused by trauma and cancerous tumors. These repairs can improve the patient's physiological abilities, such as chewing and speaking, and alleviate some of the psychological issues associated with maxillofacial injuries [183].
Silicon-based materials have found widespread use in the cosmetics industry, serving as suspending agents and playing a crucial role in processes such as emulsification and associative thickening. These materials, including silicon and its composites such as biomethane and cyclomethycaine, are commonly employed in the production of various cosmetic products, including deodorants, shampoos, antiperspirants, and lotions [184].
Polyethylene glycols (PEGs) and their non-ionic or anionic derivatives are widely utilized in cosmetics for their emulsifying and moisturizing properties. They can enhance penetration, facilitating the delivery of other active ingredients deeper into the skin. These compounds find applications in a variety of cosmetic products, including skincare items, makeup, cleansers, bath products, deodorants, hair conditioners, shampoos, and shaving products [185].
In a study by Poomanee et al. (2020), efforts were made to enhance the stability and skin penetration of Mangifera indica L. kernel extract for its potential as an effective anti-acne treatment [37]. Nanoemulsion formulations were developed using a combination of ingredients such as PEG-7 glyceryl cocoate, butylated hydroxytoluene, PEG-40 hydrogenated castor oil, ceteareth-20, safflower oil, and sorbitan oleate as the oil phase. This innovative approach resulted in the creation of a nanoemulsion that exhibited not only physicochemical stability but also demonstrated antibacterial activity and improved skin permeability of the M. indica kernel extract.
Aliphatic polyesters, including poly (lactic acid) (PLA), poly(3-hydroxybutyrate-co-3-hydroxyvalerate), and poly (ε-caprolactone) (PCL), exhibit exceptional characteristics such as mass production, biocompatibility, and biodegradability. These materials possess the advantages of being easily melted for processing and demonstrating superior mechanical properties, features not commonly found in natural polymers like gelatin, cellulose, and chitin. The unique attributes of aliphatic polyesters have garnered significant interest in their application for creating microbeads used in sustainable cosmetics [186].
Expanded polytetrafluoroethylene (ePTFE), characterized by its robust physical and chemical stability, serves as an inert polymeric tissue filler in medical applications, particularly in cosmetic surgeries [187]. This material exhibits favorable attributes that contribute significantly to its utility in these procedures. Notably, ePTFE provides optimal environments conducive to cell proliferation. Post-implantation analysis via histology reveals increased number of tissue cells and giant cells on the material's surface, along with pore filling by a collagen matrix, attachment of fibroblasts and functional capillaries to the material's exterior, and tissue integration onto the material's surface while growing within its pores. Additionally, ePTFE boasts strong mechanical properties, excellent clinical adaptability, and commendable biocompatibility [188].
Hydroxyapatite is a biocompatible material that is well-suited for use in medical applications. When implanted in the body, the calcium and phosphate ions present in hydroxyapatite are released and contribute to the balance of calcium and phosphorus in human tissues. This process occurs without causing any adverse reactions or repelling the surrounding tissues. Hydroxyapatite is commonly used in plastic surgery to replace large areas of bone, particularly in the oral and maxillofacial regions, as well as for dental implants [188]. However, pure hydroxyapatite has some limitations, including high brittleness, low strength, and poor toughness, which can limit its effectiveness as a replacement for human hard tissue. To overcome these limitations, composite materials can be created by combining hydroxyapatite with other materials. This can significantly improve the overall performance of hydroxyapatite and make it a more viable option for medical applications [188].
8.3. Exosome and Stem Cell
Emerging innovations in aesthetic medicine include exosome therapy and stem cell therapy, which demonstrate promising, minimally invasive approaches [189]. Preliminary clinical trials have indicated their effectiveness in addressing conditions such as androgenetic alopecia and stimulating skin regeneration through the employment of stem cells and conditioned medium derived from these cells. However, extensive translation into human populations remains limited at this stage. Nonetheless, initial in vitro and in vivo experiments hint towards the potential of exosome treatment for combating hair loss and revitalizing skin. The implementation of stem cells and exosomes in cosmetic procedures faces challenges due to the lack of uniform protocols and robust randomized controlled studies [190].
Deriving exosomes from adult or mesenchymal stem cells (MSCs) could introduce a groundbreaking, minimally invasive approach to skin revitalization, potentially surpassing traditional surgical methods [189]. Preliminary in vitro and in vivo studies demonstrate promising outcomes regarding photoaging alleviation, despite remaining at an early stage of investigation. Notably, these MSC-exosome findings reveal enhanced fibroblast motility and multiplication, stimulated neovascularization, and triggered endogenous stem cell mobilization – all contributing to improved tissue restoration and injury healing. Recent investigations involving exosomes originating from younger mice showcase the ability to reverse age-associated chemical expressions and gene profiles linked to telomerase in aged mouse models. This discovery holds great significance in understanding the potential of MSC-exosomes in combating the effects of aging on skin health [191].
Undoubtedly, ESCs and exosomes are becoming more popular in aesthetics [189]. Although preclinical and clinical evidence is encouraging, their function in cosmetics is still not entirely understood. There are not enough large, randomized controlled trials [189]. In addition, there is a great deal of variability in the research due to variations in the molecular phenotype and purity of the stem cell and exosome sources. Since there are hundreds of cells in the experimental products, their variability makes it challenging to evaluate their true efficacy. Furthermore, the microenvironment, which is difficult to manage, frequently modifies their effects. The recent preclinical and early clinical research will surely open the door for more sophisticated clinical trials that will decide the fate of SCs and exosomes in the field of aesthetics [192].
CONCLUSION AND FUTURE PERSPECTIVES
To sum up, the convergence of cosmetics and tissue engineering is not only a breakthrough but also a whole different transformation. The current study highlights how innovations like engineered biological products, specific biomaterials, and personalized approaches are paving the way for safer, more effective, and ethical cosmetic innovations and applications. The rapid advancements in tissue engineering for cosmetics and dermatology make it further necessary to establish and update the ethical considerations that provide the essential responsible development and clinical application [207]. The subjects, including cellular and material sources, safety and risk management, accessibility, and equity, require more ethical clarity [207, 208]. It is crucial to fully document the evidence and publish it under a responsible organization in order to facilitate the acceptance of novel products and approaches in the fields of tissue engineering for cosmetics and dermatology by society.
By fusing cutting-edge biomaterials and scaffold designs with advanced cell-based methods, a new realm has been created where cosmetic science not only deals with aging, scarring, and pigmentation challenges but also covers animal-friendly and eco-conscious practices. Rapid developments in genome editing, cell engineering, bioprinting, artificial intelligence-driven personalization, and sustainable manufacturing mean that the next wave of research should improve skin and beauty care to hitherto unheard-of degrees [35, 209, 210]. Innovative techniques like 3D bioprinting, CRISPR, and single-cell technology support these assurances [209-211]. These technologies have the potential to revolutionize the way cosmetic products are developed and have opened up a world of possibilities for personalized skincare solutions. Therefore, future prospects are extremely bright.
However, there are significant approaches based on regenerative medicine regarding its use in skincare and dermatology; several issues limit the translation of approaches and products from bench to bedside. Subsequent to successful outcomes in in vitro phases, innovative approaches encounter more preclinical considerations like safety, formulation, dosage, administration route, time of delivery, and pharmacokinetics that must be addressed before starting human clinical trials. Overcoming technical, financial, and ethical obstacles will be spurred by multidisciplinary cooperation. In summary, as advancements progress, tissue engineering is poised to transform the concept of beauty and dermatology, rendering it more innovative, sustainable, and aligned with the values of contemporary society. It is imperative that we continue to advance this trend of innovation in order to reshape the future landscape of cosmetic science and regenerative medicine.
AUTHORS’ CONTRIBUTIONS
The authors confirm their contribution to the paper as follows: A.G., P.R.: Study conception and design; Z.P.: Data collection; M.R., M.S.C., F.S., M.R.: Draft manuscript; All authors reviewed the results and approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| HA | = Hyaluronic acid |
| P&G | = Procter & Gamble |
| IND | = Investigational new drug |
ACKNOWLEDGEMENTS
Declared none.


