All published articles of this journal are available on ScienceDirect.
Annular Lichen Planus in a Patient with Ulcerative Colitis: A Case Report of a Rare Morphological Variant Associated with Systemic Disease
Abstract
Introduction/Background
Annular lichen planus (ALP) is a rare morphological variant of lichen planus that can mimic other annular dermatoses and often poses diagnostic challenges. Although lichen planus has been sporadically linked to systemic diseases, its association with ulcerative colitis is exceedingly rare. This case highlights the coexistence of ALP and ulcerative colitis, suggesting a possible immunopathogenic connection between the two conditions.
Case Presentation
A 40-year-old woman with a three-year history of ulcerative colitis presented with a six-month history of intensely pruritic, violaceous, annular lesions affecting the dorsal feet, hands, wrists, and face. Examination revealed flat-topped papules, some forming plaques with Wickham striae and annular configurations. Histopathology confirmed the diagnosis of annular lichen planus. The patient had no other known triggers such as new medications, infections, or contact allergens. She was treated with topical corticosteroids and antihistamines, resulting in partial resolution of the lesions over a four-week period.
Conclusion
This case underscores the importance of considering systemic autoimmune diseases like ulcerative colitis in patients presenting with atypical forms of lichen planus. It adds to the limited literature documenting ALP as a possible cutaneous manifestation associated with inflammatory bowel disease.
1. INTRODUCTION
Lichen planus (LP) is a chronic inflammatory disease affecting the skin and mucous membranes, with a prevalence of approximately 1% in the general population. It typically presents as pruritic, violaceous, flat-topped papules, most often involving the wrists, lower back, and ankles. While its exact pathogenesis remains unclear, LP is believed to be a T-cell-mediated autoimmune reaction, potentially triggered by infections (e.g., hepatitis C, SARS-CoV-2), medications, or contact allergens [1-6].
Annular lichen planus (ALP) is a rare clinical variant, accounting for about 10% of LP cases, and may pose diagnostic challenges due to its atypical morphology. Although associations between LP and inflammatory bowel disease (IBD) have been rarely documented, emerging reports suggest a potential link [7-10].
We present a case of annular lichen planus in a patient with underlying ulcerative colitis, highlighting a rare clinical variant and its possible association with IBD.
2. CASE REPORT
A 40-year-old female was admitted to the Clinic of Dermatology and Venereology with a six-month history of an intensely pruritic rash, initially localized to the dorsal aspect of the feet. Over the following three months, the lesions gradually spread to the dorsal surfaces of the hands, wrists, and face. One day prior to admission, the patient noted the sudden appearance of painful sores on the dorsal hands and feet.
The patient reported receiving an intravenous iron infusion shortly before the initial onset of the rash. Although the temporal proximity raised suspicion, the morphology and chronicity of the lesions, as well as the absence of recurrence following iron administration, made iron-induced lichenoid eruption unlikely. The patient had a known history of ulcerative colitis, managed with oral mesalazine (Salofalk) for the past three years. She denied any other new medications or recent infections.
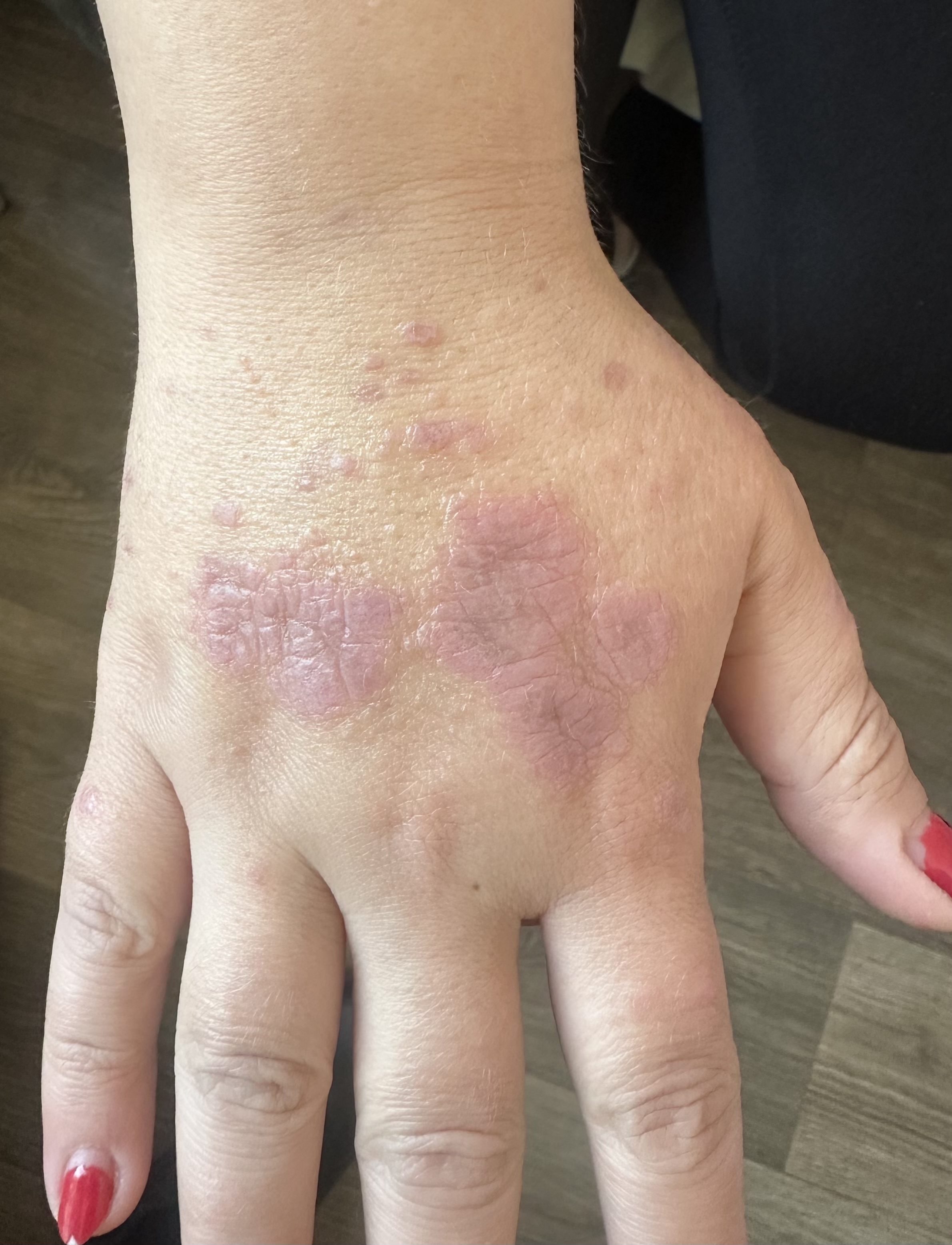
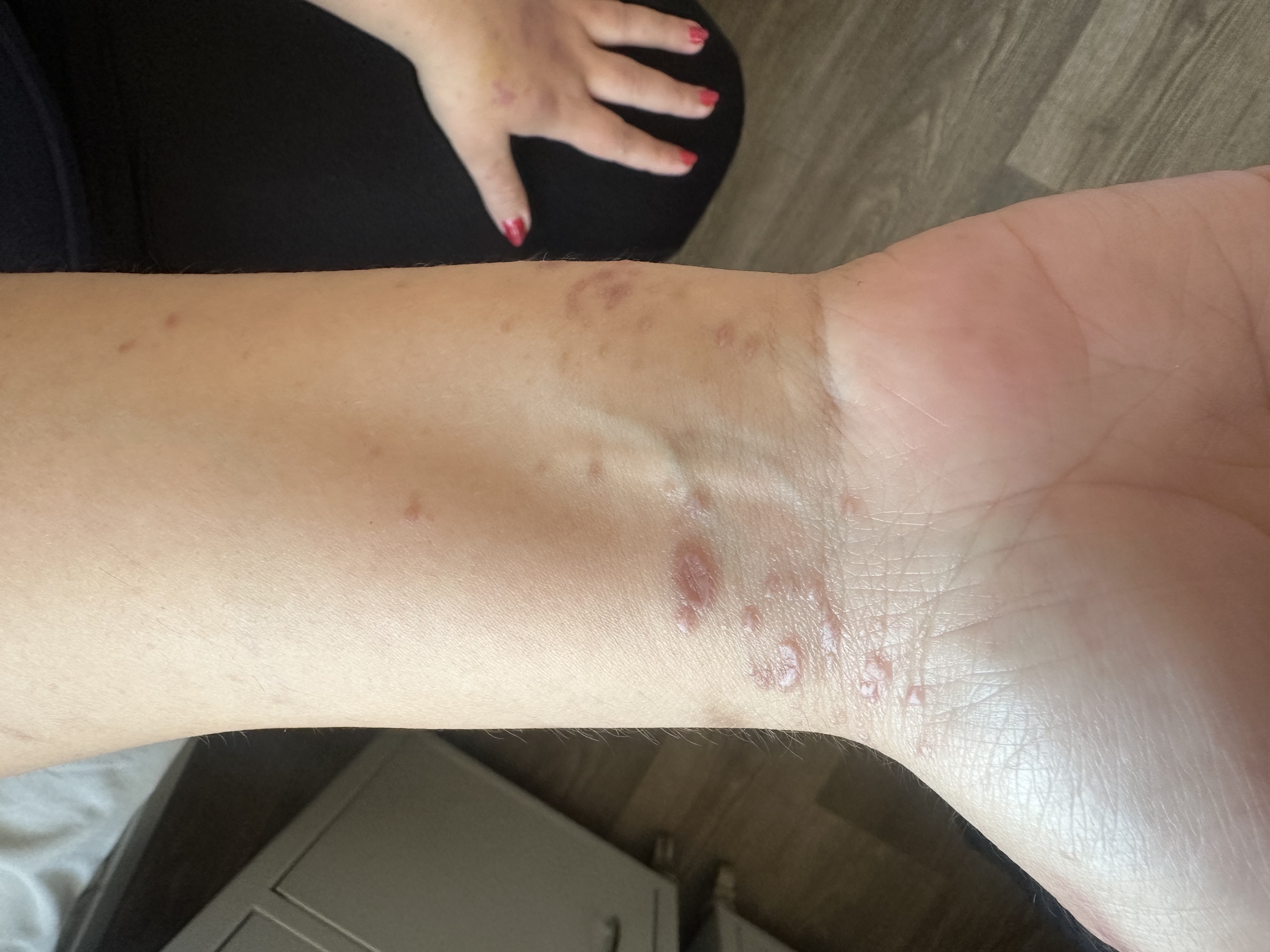
Physical examination revealed multiple erythematous to violaceous, flat-topped, polygonal papules on the anterior wrists, some coalescing into plaques with fine white lines (Wickham striae) (Fig. 1). On the dorsal hands, violaceous papules merged into annular plaques with raised borders and central clearing (Fig. 2). A distinctive circular configuration of confluent lichenoid papules was noted on the left dorsal hand. The dorsal feet showed hyperpigmented papules and plaques, also marked by Wickham striae. The facial lesions consisted of hyperpigmented plaques accompanied by mild erythema.
Flat-topped, polygonal, violaceous papules with Wickham striae on the anterior wrists, consistent with classic lichen planus lesions.
Annular violaceous plaques with raised borders and central clearing on the dorsal hands, characteristic of annular lichen planus.
2.1. Timeline
- 3 years ago: Diagnosis of ulcerative colitis; treatment with oral mesalazine (Salofalk) initiated.
- 6 months prior to admission: Onset of intensely pruritic rash on the dorsal feet.
- 3 months prior to admission: Lesions spread to the hands, wrists, and face.
- 1 day prior to admission: Sudden appearance of painful sores on the dorsal hands and feet.
- Day of admission: Clinical examination performed; biopsy taken from dorsal hand.
- Weeks 1–4: Treated with topical corticosteroids and oral antihistamines; partial improvement observed.
- Post-treatment: Patient did not return for scheduled follow-up; long-term outcome remains unknown.
3. METHODOLOGY
A clinical diagnosis of annular lichen planus was based on the patient’s characteristic morphology—violaceous, flat-topped papules with Wickham striae, forming annular plaques with raised borders and central clearing. Dermoscopic examination revealed Wickham striae and a peripheral violaceous pigment network. A 4 mm punch biopsy was performed from a representative lesion on the dorsal hand. Histopathology confirmed the diagnosis by revealing a dense band-like lymphocytic infiltrate at the dermo-epidermal junction, basal cell degeneration, and the presence of Civatte bodies. Routine laboratory tests were conducted to exclude systemic infection or other autoimmune conditions. The patient was prescribed a high-potency topical corticosteroid (clobetasol propionate 0.05%) applied twice daily to the affected areas. In addition, an oral antihistamine was given to help manage pruritus. After four weeks of therapy, partial clinical improvement was observed, with reduced erythema and flattening of the plaques. However, the patient did not return for scheduled follow-up, and long-term outcomes remain unknown. The patient provided written informed consent for publication.
4. DISCUSSION
Lichen planus (LP) is a chronic inflammatory disorder that primarily affects the skin and mucous membranes and affects approximately 0.5-1% of the population [1]. Clinically, it presents as intensely pruritic, violaceous, flat-topped papules and plaques, commonly localized to the wrists, lower back, and ankles. A hallmark diagnostic feature is the presence of Wickham striae—a fine, white, reticulated pattern on the lesion surface—resulting from hypergranulosis of the epidermis [2].
Although the exact pathogenesis of LP remains unclear, it is widely considered a T-cell-mediated autoimmune disease that targets basal keratinocytes. A variety of potential triggers have been implicated, including viral infections (notably hepatitis C virus and, more recently, SARS-CoV-2 and its vaccines), drugs (such as antimalarials, nonsteroidal anti-inflammatory drugs, beta-blockers, and TNF-alpha inhibitors), and contact allergens like metals used in dental restorations [3-6].
The association between LP and inflammatory bowel disease (IBD), while not as well-established, is biologically plausible. IBD is frequently accompanied by extraintestinal manifestations, particularly dermatologic ones. Given the shared immune-mediated mechanisms in both conditions, a pathogenic link between LP and IBD—especially ulcerative colitis—has been suggested in a few case reports and small studies [7-11]. In the present case, the absence of other identifiable triggers points to ulcerative colitis as the most likely underlying factor contributing to the development of annular LP.
ALP, a morphological variant of LP, is typically characterized by annular plaques with raised violaceous borders and central clearing. Unlike classic LP, where violaceous, flat-topped papules dominate the clinical picture, ALP normally presents only with annular lesions and rarely coexists with the classic papular form [7, 12]. Though it usually appears in the genital or intertriginous regions, our patient exhibited widespread involvement, along with classical LP lesions and Koebner phenomenon (Fig. 3).
The primary differential diagnosis was granuloma annulare (GA), as both conditions can present with annular plaques and central clearing. However, GA lesions tend to be skin-colored or erythematous, lack Wickham striae, and are usually asymptomatic [13]. In contrast, our patient's intensely pruritic, violaceous lesions with histologic confirmation supported ALP. Tinea corporis/ faciei and subacute cutaneous lupus erythematosus were also considered but ruled out based on morphology, lack of scale, serology, and histopathological findings.
The coexistence of ulcerative colitis and ALP, in the absence of other identifiable triggers, suggests a possible immunopathogenic connection. One hypothesis is that systemic immune dysregulation in ulcerative colitis may promote lichenoid reactions in the skin, although the exact mechanism remains unclear [14].
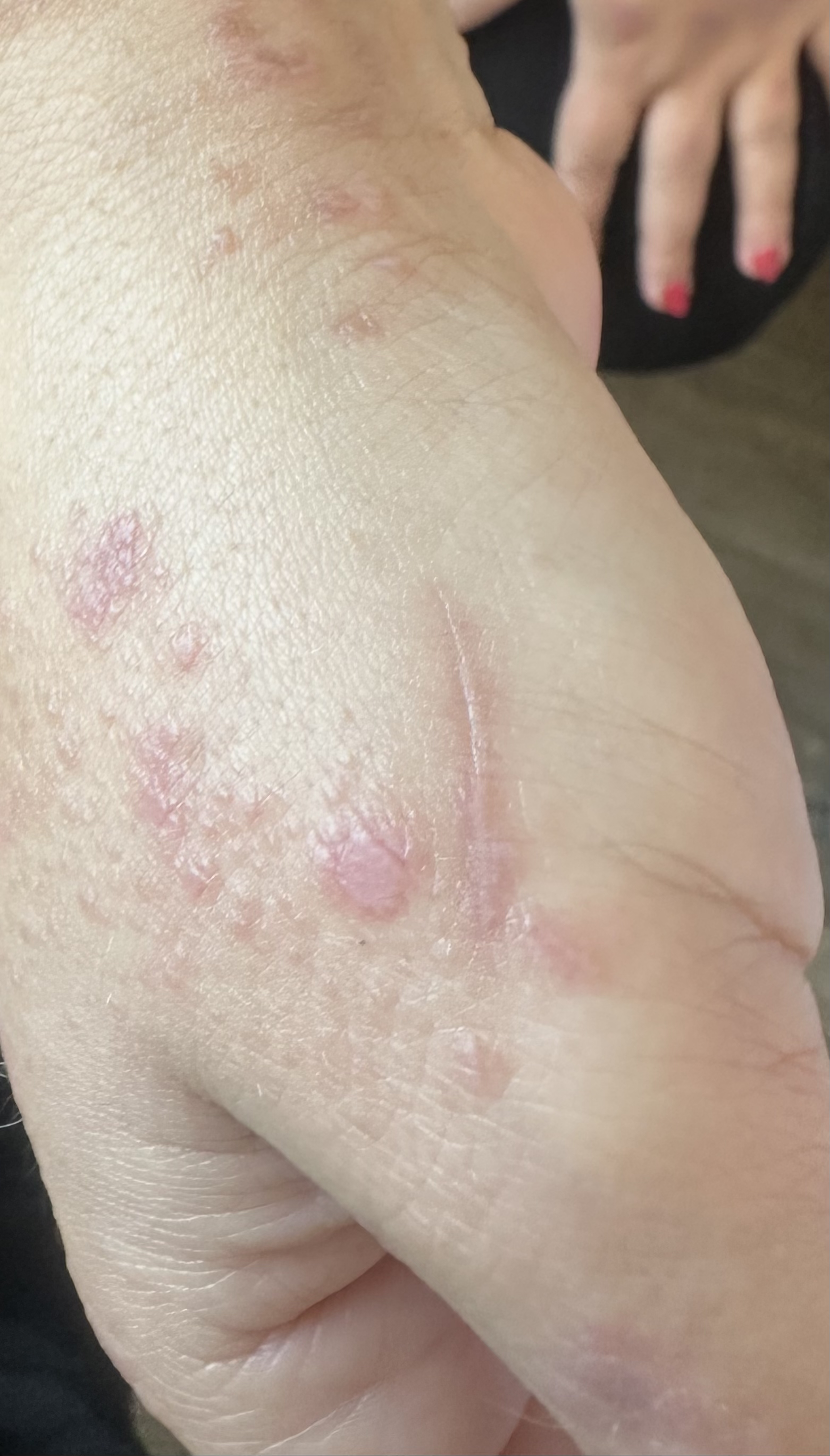
Classical LP papules with Koebner phenomenon on sites of trauma, supporting diagnosis.
CONCLUSION
Annular lichen planus is a rare clinical variant that may coexist with classic LP lesions and can mimic other annular dermatoses, making diagnosis challenging. Its potential association with systemic diseases such as ulcerative colitis should prompt clinicians to consider immunological comorbidities in patients presenting with atypical dermatologic findings.
This case is particularly notable due to the coexistence of ALP and ulcerative colitis, with no other identifiable triggers. While extraintestinal manifestations of IBD are well-recognized, cutaneous involvement with ALP remains rare. Our observations support a potential immune-mediated connection between ulcerative colitis and annular lichen planus. However, as this is a single-patient case report, causality cannot be established, and the lack of long-term follow-up and additional immunological investigations limits the strength of our conclusion. Further reports and larger studies are necessary to better understand and validate this possible association.
PATIENT PERSPECTIVES
The patient described the skin condition as both physically uncomfortable and emotionally distressing due to its visible appearance. She expressed relief upon receiving a clear diagnosis and appreciated the prompt initiation of treatment.
AUTHORS’ CONTRIBUTIONS
The authors confirm their contribution to the paper as follows: study conception and design: TA, VG; data collection: VG; analysis and interpretation of results: TA, VG, MC; draft manuscript preparation: TA, MC. All authors reviewed the results and approved the final version of the manuscript.
AVAILABILITY OF DATA AND MATERIAL
The data supporting the findings of this case report, including anonymized clinical images and timeline summary, are available in the Zenodo repository at https://doi.org/10.5281/zenodo.16750559, reference number: 10.5281/zenodo.16750559.
ACKNOWLEDGEMENTS
The authors would like to thank the patient for providing consent to share her case for educational and scientific purposes.


