All published articles of this journal are available on ScienceDirect.
Mechanical Stretching Modulates PGE2 and TGF-β1 Levels in Human Keratinocytes
Abstract
Background
Mechanical stresses affect biological processes, cellular homeostasis, and skin wound healing.
Objective
We investigated the effects of mechanical stretching on epidermal keratinocytes, focusing on its role in wound healing and keloid formation.
Methods
Epidermal keratinocytes were stretched using a biochemical stretch device with or without a cyclooxygenase-2 inhibitor. Cells and supernatants were collected after 1−24 h of stimulation. Stretched keratinocytes were co-cultured with normal dermal or keloid-derived fibroblasts. Prostaglandin E2 and 12-hydroxyeicosatetraenoic acid were measured via enzyme-linked immunosorbent assay. Prostaglandin E2 receptor EP3 and transforming growth factor-β1 (TGF-β1) were analyzed using qRT-PCR. TGF-β1 was also examined using western blotting. Prostaglandin E2 synthase and TGF-β1 were also stained and analyzed using immunofluorescence microscopy.
Results
Mechanical stretching of keratinocytes increased the levels of prostaglandin E2, which stimulates keratinocyte proliferation and exerts antifibrotic effects, as well as those of prostaglandin E synthase 2 and prostaglandin E2 receptor 3. In contrast, mechanical stretching did not affect the levels of transforming growth factor-β1 in affected keratinocytes. However, co-culture with keloid-derived fibroblasts markedly increased the levels of transforming growth factor-β1 in mechanically stretched keratinocytes.
Discussion
These results suggest that the stretching stimulation of keratinocytes promotes epithelialization by increasing prostaglandin E2 production. Furthermore, stretching stimulation of keratinocytes in the presence of keloid-derived fibroblasts possibly promotes keloid formation via the upregulation of TGF-β1 levels in keratinocytes.
Conclusion
Mechanical stretching induced PGE2 production and increased the TGF-β1 levels in keratinocytes in the presence of keloid-derived fibroblasts. Our results suggest that stretch stimulation in the presence of keloid-derived fibroblasts promotes keloid development.
1. INTRODUCTION
Cells are constantly exposed to various mechanical stresses, including gravity, compression, and stretching. Mechanical stress affects cell homeostasis and various biological processes, including skin wound healing, in the human body. Contraction of fibroblast–collagen matrix induces isometric tension in fibroblasts and stimulates extracellular signal-regulated kinase (ERK) and p38 mitogen-activated protein kinases [1]. Mechanical stretching promotes human keratinocyte proliferation by inducing calcium influx, phosphorylation of the epidermal growth factor receptor, and ERK1/2 cascades [2]. Mechanical stretching also causes epithelial–mesenchymal transition and promotes epidermal restoration by controlling keratinocyte differentiation and proliferation [3]. Negative pressure wound therapy (NPWT) applies negative pressure to wounds, sealing them, removing any exudates, accelerating wound contraction and granulation, and facilitating wound healing by promoting epidermal basal cell proliferation. Moreover, NPWT elevates the transforming growth factor (TGF)-β1 levels in diabetic ulcers [4, 5]. However, the specific mechanisms by which NPWT accelerates wound healing remain unknown. Wound immobilization and compression effectively prevent scar formation because repeated wound stretching leads to keloid formation. Fibrosis is characterized by the presence of smooth muscle actin-positive myofibroblasts originating from fibroblasts and mesenchymal stem cells, which cause excess deposition of extracellular matrix and tissue contraction, and can occur in any organ during the remodeling of damaged tissue. Chronic inflammation leads to abnormal remodeling. Unlike epithelialization, dermal fibrosis is primarily caused by fibroblasts, which reside in the dermis and generate extracellular matrix. Mechanical stress on fibroblasts may be involved in skin fibrosis; however, its effects on epidermal keratinocytes and its role in skin fibrosis remain unclear. In this study, we aimed to elucidate whether stretching stimulation of epidermal keratinocytes affects wound healing and keloid formation, focusing on prostaglandin E2 (PGE2) and TGF-β1, which are involved in wound healing.
2. MATERIALS AND METHODS
2.1. Cell Culture
Primary normal human epidermal keratinocytes (NHEKs; 3.0 × 105 cells/10 cm dish) (Kurabo, Osaka, Japan) were cultured in the keratinocyte growth medium 2 (KG2) (HuMedia-KG2; Kurabo) supplemented with insulin, human epidermal growth factor, hydrocortisone, and bovine pituitary extract in a humidified incubator at 37 °C and 5% CO2. The culture medium was changed twice a week. NHEKs at passages 2–3 were used in subsequent experiments.
NHEKs were also cultured in KG2 containing 5 μM celecoxib, a cyclooxygenase (COX)-2 inhibitor, for 24 h, to examine COX2-inhibited keratinocytes after stretching stimulation.
After obtaining informed consent, keloid fibroblasts were obtained from keloid tissues at the time of surgical excision. Cells were maintained in Dulbecco's modified Eagle's medium (DMEM) (Sigma, St. Louis, MO, USA) containing 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin (Wako, Osaka, Japan). Normal skin specimens were obtained during other operations.
2.2. Cell Stretching
NHEKs (2.0 × 105 cells/well) were seeded in a four-well flexible silicone chamber. Before mechanical stretching, NHEKs were cultured in KG2 medium for 24 h. The cells were then stretched using a biochemical stretch device (NST-1400; NEPAGene, Chiba, Japan) at 20% extension (30 rpm)2,27. The device was operated at 37 °C and 5% CO2 during stretching stimulation. Cells and cell culture supernatants were harvested after 1, 3, 6, and 24 h of stretching. The control cells were cultured on the same plate and incubated without mechanical stretching.
2.3. Co-culture
Normal human dermal fibroblasts (NHDFs) or keloid-derived fibroblasts (5 × 104 cells) at passages 3–6 were seeded in the bottom chamber of a six-well plate (IWAKI, Shizuoka, Japan) and cultured in the Dulbecco’s modified Eagle’s medium (Sigma) containing 10% fetal bovine serum and 1% penicillin–streptomycin (Wako) for 24 h. Meanwhile, stretched or non-stretched NHEKs (1 × 105 cells) were seeded on a 0.4-μm pore size cell culture insert (cellQART, Northeim, Germany) in KG2 and cultured for 24 h.
2.4. Enzyme-linked Immunosorbent Assay (ELISA)
Cell culture medium was harvested from each plate after 6 h of stretching stimulation to determine PGE2 and 12-hydroxyeicosatetraenoic acid (12-HETE) levels. PGE2 levels in the culture medium were measured using an ELISA kit (Cayman Chemical, Ann Arbor, MI, USA), according to the manufacturer’s instructions. Absorbance of the extracted supernatant was measured using a microplate reader (λ = 405 nm; Thermo Fisher Scientific, Waltham, MA, USA) with a standard.
2.5. RNA Extraction and Reverse Transcription-quantitative Polymerase Chain Reaction (RT-qPCR)
Cells were lysed, and mRNA was isolated using the RNeasy Mini Kit (QIAGEN, Hilden, Germany) and reverse transcribed into cDNA using the ReverTra Ace qPCR RT Kit (TOYOBO, Osaka, Japan). RT-qPCR was performed using the TaqMan Fast Universal PCR Master Mix (TOYOBO) with the Step One real-time PCR system (Thermo Fisher Scientific). The following genes were analyzed using TaqMan Gene Expression Assays (Thermo Fisher Scientific): PGE2 receptor 3 (EP3; Hs00168755_ml) and TGF-β1 (Hs00998133_ml). Relative expression was calculated using the ΔΔCt method, with glyceraldehyde 3-phosphate dehydrogenase (607901; BioLegend) as the internal reference gene.
2.6. Western Blotting Analysis
The cells were homogenized and lysed on ice using radioimmunoprecipitation assay buffer (Cell Signaling Technology). The cell lysates were centrifuged at 14,000 × g for 10 min at 4 °C. The supernatants were harvested, and equal amounts of protein were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes. The membranes were then incubated with anti-human TGF-β1 primary antibody (Abcam, Cambridge, UK) at 4 °C overnight. After washing with 0.1% Tween-20 in phosphate-buffered saline (PBS), the membranes were incubated with a horseradish peroxidase-conjugated anti-rabbit immunoglobulin G (IgG) antibody (Cell Signaling Technology). Subsequently, the protein bands were visualized using the Super Signal West Dura Extended Duration Substrate (Thermo Fisher Scientific) with ImageQuant LSA4000 (Cytiva, Tokyo, Japan). The band intensity was quantified using a Multi Gauge (Fuji Film, Tokyo, Japan). β-actin (664801; Bio Legend) was used as the internal control. Densitometry analysis was performed using ImageJ software (National Institutes of Health).
2.7. Cell Imaging
After fixing with 4% paraformaldehyde for 15 min at room temperature, the cells were blocked with PBS containing 0.1% Triton-X 100 (Sigma-Aldrich Inc, St. Louis, MO) and 10% normal goat serum (Jackson ImmunoResearch Laboratories, West Grove, PA) for 45 min at room temperature, and incubated with primary antibodies against prostaglandin E synthase (PGES)-2 (bs-2639R; Bioss) and TGF-β1 (EPR21143; Abcam) for 45 min at 37 °C. The cells were then washed, incubated with goat anti-rabbit IgG coupled with Alexa Fluor 488 (1:500; Life Technologies) secondary antibody for 45 min at room temperature in the dark, and washed three times with PBS. Cell nuclei were stained with ProLong Diamond (Thermo Fisher Scientific) after incubation with the secondary antibody. Digital images were captured using an EVOS M5000 fluorescence microscope (Thermo Fisher Scientific). To determine the amount of fluorescence and compare it with that of the controls, fluorescent cells were automatically counted to assess the corrected total cell fluorescence (CTCF) using the ImageJ software (National Institutes of Health).
2.8. Statistical Analysis
Results are presented as the mean ± standard error of the mean of at least three separate experiments, and each experiment was performed in triplicate. Statistical analysis of two groups was performed using the Student’s t-test, whereas that of multiple groups (2 E and F) was performed using analysis of variance followed by Tukey’s test.
3. RESULTS
3.1. Mechanical Stretching increases the PGE2 Levels in NHEKs
An enzyme-linked immunosorbent assay (ELISA) using cell culture supernatants was performed to investigate the effect of mechanical stretching on PGE2 secretion from keratinocytes. Mechanical stretching considerably increased PGE2 levels (Fig. 1A). We investigated PGES2 expression using immunofluorescence microscopy. PGES2 expression was notably increased by mechanical stretching (Fig. 1B). Moreover, the CTCF of PGES2 in stretched NHEKs was significantly higher than that in non-stretched NHEK (Fig. 1C).
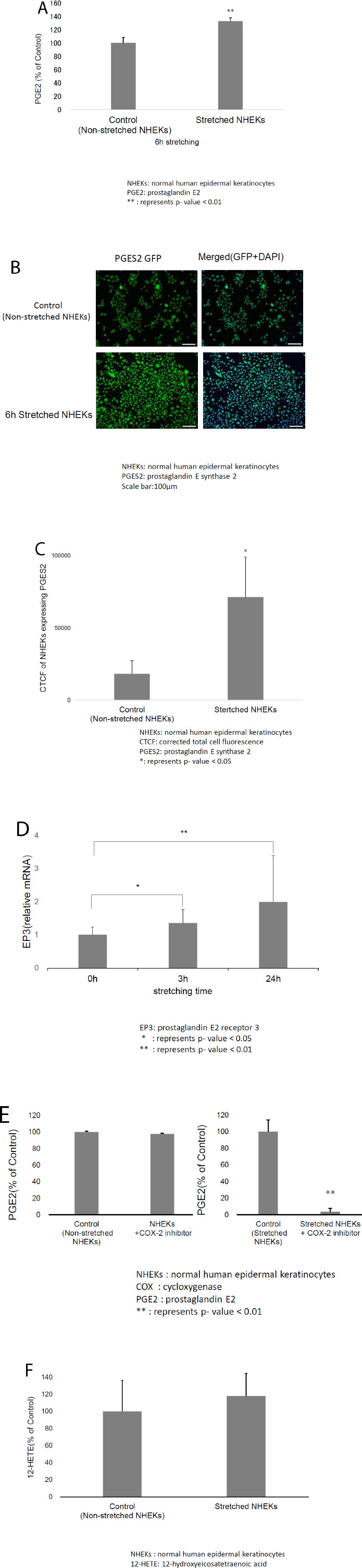
A) Enzyme-linked immunosorbent assays (ELISA) revealed that mechanical stretching considerably increased prostaglandin E2 (PGE2) levels in normal human epidermal keratinocytes (NHEKs). B) Immunofluorescence microscopy revealed that the prostaglandin E synthase 2 (PGES2) expression in NHEKs was significantly increased by mechanical stretching. C) The corrected total cell fluorescence (CTCF) of stretched NHEKs expressing PGES2 was significantly higher than that of non-stretched NHEKs. D) Mechanical stretching increased EP3 mRNA levels in NHEKs over time. E) ELISA revealed that the cyclooxygenase 2 (COX-2) inhibitor did not affect PGE2 levels in NEHK, but suppressed the mechanical stretch-induced increase in PGE2 levels. F) ELISA revealed that mechanical stretching increased 12-hydroxyeicosatetraenoic acid (12-HETE) in NHEKs, although this difference was not statistically significant.
3.2. Mechanical Stretching increases the EP3 mRNA Level in NHEKs
We measured EP3 mRNA levels to further assess the effects of mechanical stretching on keratinocytes. Mechanical stretching increased the EP3 mRNA levels in NHEKs over time (Fig. 1D). This result suggested that mechanical stretching of keratinocytes promotes epithelialization by increasing PGE2 levels.
3.3. COX-2 Inhibition decreases the PGE2 Levels increased by Mechanical Stretching
We investigated whether the mechanical stretching-induced increase in PGE2 levels was blocked by a COX-2 inhibitor to confirm the effects of mechanical stretching on keratinocytes. After incubation with a COX-2 inhibitor, keratinocytes were mechanically stretched. ELISA revealed that the COX-2 inhibitor did not affect PGE2 levels in NEHKs but suppressed the mechanical stretching-induced increase in PGE2 levels (Fig. 1E). These results suggest that mechanical stretching not only increases the PGES2 levels but also promotes the release of arachidonic acid from keratinocyte cell membranes.
We investigated changes in the levels of 12-HETE, a major metabolite of arachidonic acid, to confirm the effects of mechanical stretching on the release of arachidonic acid from keratinocytes. ELISA revealed that mechanical stretching did not significantly increase the 12-HETE levels in NHEK cells (Fig. 1F).
3.4. Mechanical Stretching does not Affect the TGF-β1 Levels in NHEKs
Next, we determined the TGF-β1 mRNA levels in keratinocytes. Mechanical stretching did not affect the TGF-β1 mRNA levels in NHEKs (Fig. 2A). Immunofluorescence microscopy showed that TGF-β1 levels in NHEKs did not increase after mechanical stretching (Fig. 2B). Moreover, CTCF of stretched NHEKs expressing TGF-β1 was not significantly different from that of non-stretched NHEKs (Fig. 2C).
Western blotting confirmed that mechanical stretching did not affect the TGF-β1 levels in keratinocytes (Fig. 2D). These results suggested that mechanical stretching was not directly associated with keloid formation.
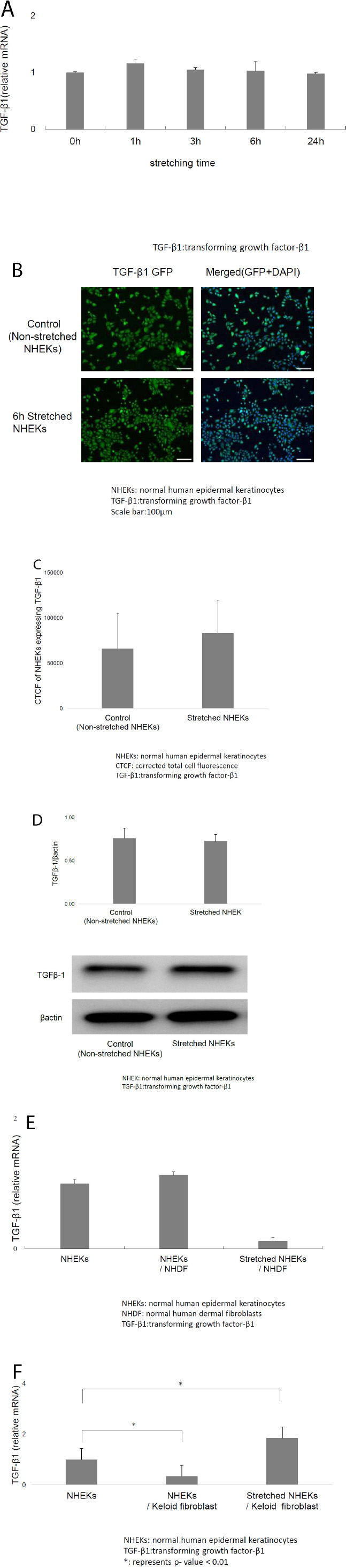
A) Mechanical stretching did not affect transforming growth factor (TGF)-β1 mRNA levels in NHEKs. B) Immunofluorescence microscopy revealed that TGF-β1 expression in NHEKs did not increase following mechanical stretching. C) CTCF of stretched NHEKs expressing TGF-β1 showed no significant differences compared with non-stretched NHEKs. D) Western blotting also revealed that mechanical stretching did not affect TGF-β1 expression in keratinocytes. E) Mechanical stretching suppressed TGF-β1 mRNA level in NHEKs co-cultured with normal dermal fibroblasts, but no significant difference was observed. F) Mechanical stretching considerably increased TGF-β1 mRNA levels in NHEKs co-cultured with keloid-derived fibroblasts.
3.5. Mechanical Stretching increases the TGF-β1 Levels in NHEKs Co-cultured with Keloid-derived Fibroblasts
Mechanical stretching non-significantly decreased the TGF-β1 mRNA levels in NHEKs co-cultured with normal dermal fibroblasts (Fig. 2E). In contrast, mechanical stretching significantly increased the TGF-β1 mRNA levels in NHEKs co-cultured with keloid-derived fibroblasts (Fig. 2F). Therefore, combined stretching stimulation and keloid fibroblast co-culture induced keloid formation, and mechanical stretching alone did not affect the TGF-β1 levels in keratinocytes.
4. DISCUSSION
Prostaglandins are potent eicosanoids, which comprise a group of arachidonic acid-derived lipid mediators that regulate inflammation and homeostasis. PGE2, the most abundant eicosanoid, promotes tissue repair in several organs. Dermal fibroblasts generate PGE2 [6], which considerably increases epidermal keratinocyte proliferation for wound healing [7]. PGE2 levels are higher in wound fluid than in the plasma of patients with chronic wounds [8] because PGE2 is synthesized in various cells, including monocytes and macrophages [8, 9] in the wound microenvironment. PGE2 stimulates angiogenesis via the TGF-β/Alk5 signaling pathway [10]. Although PGE2 is involved in wound healing, its mechanisms of action remain unclear.
Keratinocytes activate several autocrine signaling pathways. This study focused on the PGE2 and TGF-β1 levels in keratinocytes after stretching stimulation. Our results revealed that stretched keratinocytes are a more direct source of PGE2 than non-stretched keratinocytes. The PGE2 secreted by keratinocytes can act in an autocrine or paracrine manner. The lack of commercially available antibodies makes it difficult to measure PGE2 levels using techniques other than ELISA. Mechanical stretching increased PGE2 levels in cultured human amniotic and trabecular meshwork cells [11, 12]. However, to the best of our knowledge, this study is the first to demonstrate increased PGE2 production by mechanical stretching of human keratinocytes, which is a potential mechanism by which NPWT accelerates epithelialization.
PGE2 is a dual-function molecule that regulates both inflammation and fibrosis [13-16]. A previous study has reported that PGE2 administration during the early stages of wound healing promotes fibrosis and decreases the number of macrophages [17]. However, some studies have revealed that PGE2 exerts anti-fibrotic and protective effects on the lower airway mucosa [18-22]. Additionally, PGE2 inhibits TGF-β1-stimulated collagen production in dermal fibroblasts by regulating the balance between matrix metalloproteinases and tissue inhibitors of metalloproteinases [23]. Decreased PGE2 levels may be associated with increased fibrosis and poor tissue repair; however, the precise role of PGE2 in connective tissue repair and fibrosis remains unclear. PGE2 also reverses the TGF-β1-enhanced fibroblast-to-myofibroblast transition as an anti-fibrotic effect. It prevents keloid formation by suppressing collagen synthesis in keloid-derived fibroblasts and by inhibiting cell migration and contraction [14]. In this study, stretching increased the PGE2 production in keratinocytes. Our results suggested that epidermal stretching directly induces keloid formation during the early stages of wound healing. Moreover, co-culture with keloid-derived fibroblasts significantly increased the levels of TGF-β1, which is involved in fibroblast contraction during wound healing and keloid formation in mechanically stretched keratinocytes. Therefore, in the presence of keloid-derived fibroblasts, stretching stimulation may induce keloid development by increasing the TGF-β1 levels in keratinocytes; however, keratinocyte-derived TGF-β1 may not necessarily target fibroblasts. As mechanical stretching also promotes integrin-dependent activation of latent TGF-β1, the activation mechanism of TGF-β1 should be further investigated.
PGE2 is formed from arachidonic acid by COXs and PGES, which are regulated by exogenous factors, such as interleukin (IL)-1β [18, 24, 25]. Regulation of COX-2 by IL-1β is responsible for the integrated inflammatory response to tissue injury, which induces the production and secretion of secondary inflammatory mediators, including PGE2, facilitating wound healing [14, 26]. COX-2 expression and PGE2 secretion are increased in response to tissue damage. Here, stretching of keratinocytes did not affect the IL-1β, IL-1α, and IL-6 levels (data not shown). These results suggest that IL-1β, which regulates PGE2 production in keratinocytes, is extracellularly produced. Furthermore, COX-2 inhibition suppressed the mechanical stretching-induced PGE2 upregulation in keratinocytes. Mechanical stretching of the keratinocytes did not significantly increase the levels of 12-HETE, a major product of arachidonic acid. These data suggest that mechanical stretching increases the levels of PGES2 and releases arachidonic acid from the cell membrane, resulting in enhanced PGE2 production. Additionally, COX-2 inhibitors may limit stretching-induced increases in epithelialization and fibrosis during wound healing by suppressing PGE2.
Mechanical stretching promotes keratinocyte proliferation by inducing calcium influx and epidermal growth factor receptor and ERK1/2 cascades.2 It also promotes epidermal regeneration by inducing epithelial–mesenchymal transition and regulating keratinocyte proliferation and differentiation [3]. In this study, mechanical stretching affected PGE2 production by keratinocytes. Moreover, mechanical stretching directly caused keloid formation, promoted wound healing through PGE2 production, and increased keloid development by upregulating TGF-β1 in keratinocytes in the presence of keloid-derived fibroblasts.
CONCLUSION
In conclusion, this study revealed that mechanical stretching induced PGE2 production and increased the TGF-β1 levels in keratinocytes in the presence of keloid-derived fibroblasts. Our findings provide novel insights into the mechanisms underlying wound healing and NPWT. Our results suggest that stretch stimulation in the presence of keloid-derived fibroblasts promotes keloid development. Further studies are required to investigate the properties and signal transduction pathways of stretched keratinocytes.
AUTHORS’ CONTRIBUTIONS
The authors confirm their presentation to the paper as follows: J.S. Conducted the experiments, made substantial contributions to the acquisition, analysis, and interpretation of data, and wrote the paper. T.H. and S.I. Critically revised the manuscript for important intellectual content. R.W. Participated in the design of the data. All authors read and approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| TGF-β1 | = Transforming growth factor-β1 |
| ERK | = Extracellular signal-regulated kinase |
| NPWT | = Negative pressure wound therapy |
| DMEM | = Dulbecco's modified Eagle's medium |
| FBS | = Fetal bovine serum |
| CTCF | = Corrected total cell fluorescence |
| ELISA | = Enzyme-linked immunosorbent assay |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study protocol was approved by the Ethics Committee of the Juntendo University Graduate School of Medicine (E22-H21-0044), Japan.
HUMAN AND ANIMAL RIGHTS
All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or research committee and with the 1975 Declaration of Helsinki, as revised in 2013.
CONSENT FOR PUBLICATION
After obtaining informed consent, keloid fibroblasts were obtained from keloid tissues at the time of surgical excision.
AVAILABILITY OF DATA AND MATERIALS
The data and supportive information are available within the article.
FUNDING
This research was financially supported by JSPS KAKENHI Grant Number JP21K08357, and in part by a Grant-in-Aid for Special Research in Subsidies for ordinary expenses of private schools from The Promotion and Mutual Aid Corporation for Private Schools of Japan.
CONFLICT OF INTEREST
The author, Dr. Toshio Hasegawa, is a member of the Editorial Advisory Board of The Open Dermatology Journal.
ACKNOWLEDGEMENTS
The authors would like to thank Honyaku Center Inc. for English language editing.


