All published articles of this journal are available on ScienceDirect.
Sensitizing Capacities and Cross-Reactivity Patterns of Some Diisocyanates and Amines Using the Guinea-Pig Maximization Test. Can p-phenylenediamine be Used as a Marker for Diisocyanate Contact Allergy?
Abstract
Background:
Isocyanates are mainly considered respiratory allergens but can also cause contact allergy. Diphenylmethane-4,4′-diamine (4,4′-MDA) has been considered a marker for diphenylmethane-4,4′-diisocyanate (4,4′-MDI) contact allergy. Furthermore, overrepresentation of positive patch-test reactions to p-phenylenediamine (PPD) in 4,4′-MDA positive patients have been reported.
Objectives:
To investigate the sensitizing capacities of toluene-2,4-diisocyanate (2,4-TDI) and PPD and the cross-reactivity of 4,4′-MDA, 2,4-TDI, dicyclohexylmethane-4,4′-diamine (4,4′-DMDA), dicyclohexylmethane-4,4′-diisocyanate (4,4′-DMDI), 4,4′-MDI and PPD.
Methods:
The Guinea Pig Maximization Test (GPMT) was used.
Results:
PPD was shown to be a strong sensitizer (p<0.001). Animals sensitized to PPD showed cross-reactivity to 4,4′-MDA (p<0.001). Animals sensitized to 4,4′-MDA did not show cross-reactivity to PPD. 8 animals sensitized to 2,4-TDI were sacrificed due to toxic reactions at the induction site and could thus not be fully evaluated.
Conclusion:
PPD was shown to be a strong sensitizer. However, it cannot be used as a marker for isocyanate contact allergy. On the other hand, positive reactions to 4,4′-MDA could indicate a PPD allergy. The intradermal induction concentration of 2,4-TDI (0.70% w/v) can induce strong local toxic reactions in guinea-pigs and should be lowered.
1. INTRODUCTION
Diisocyanates are reactive compounds used in the production of polyurethane (PUR). PUR products are widely used and can be found in applications stretching from rigid and flexible foams to coatings, elastomers (rubber) and adhesives [1]. Diisocyanates are mainly associated with airborne occupational exposure which can lead to negative effects on the respiratory tract and cause airway disorder and asthma [2-5]. However, they can also cause contact allergy and lately the importance of the dermal exposure as possible route to isocyanate asthma has been discussed in several papers [3, 6, 7]. Allergic contact dermatitis caused by isocyanates is mainly considered to be an occupational problem and consumers are rarely exposed to isocyanates.
There are several commercially available patch-test preparations that can be used for the establishment of isocyanate contact allergy. The most common is diphenylmethane-4,4′-diisocyanate (4,4′-MDI) since it represents the most commonly used isocyanate within industry. However, patch-test preparations of 4,4′-MDI have been shown to be inadequate [8]. Therefore, the structurally related amine, diphenylmethane-4,4′-diamine (4,4′-MDA), has been suggested as a marker for 4,4′-MDI allergy [9, 10] since several reports show that workers exposed to MDI react positively to 4,4′-MDA but not to 4,4′-MDI [9-11]. This was confirmed in a recent animal study where the cross-reactivity patterns of 4,4′-MDI, 4,4′-MDA, dicyclohexylmethane-4,4′-diisocyanate (4,4′-DMDI) and dicylohexylmethane-4,4′-diamine (4,4′-DMDA) were investigated [12]. There are also reports of concomitant positive reactions to 4,4′-MDA and p-phenylenediamine (PPD) [13, 14].
The aim of this study was to investigate the sensitizing capacity of PPD and its cross-reactivity to 4,4′-MDI, 2,4-TDI, PPD, 4,4′-DMDI, 4,4′-MDA and 4,4′-DMDA using the guinea-pig maximization test (GPMT). All investigated substances are specified in Table 1.
| Name§ | Synonyms | CAS-no | Structure | Harmonized Classification§§ | log Po/W |
Purity* (%) |
||
|---|---|---|---|---|---|---|---|---|
| Class and Category Code | Hazards Statement Code | Specific Concentration Limits | ||||||
| Diphenylmethane-4,4'-diisocyanate | 4,4′-MDI; 4,4'-Diisocyanatodiphenylmethane; 4,4'-Methylenebis(phenyl isocyanate); 4,4'-Methylenediphenyl diisocyanate | 101-68-8 | 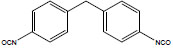 |
Skin Sens. 1 Resp. Sens. 1 Carc. 2 |
H317 H334 H351 |
Resp. Sens. 1; H334: C ≥ 0.1% | 5.22 | 98% |
| Diphenylmethane-4,4'-diamine | 4,4′-MDA; 4,4'-Methylenedianiline; 4,4'-Dimethylenediamine; 4,4'-Diaminodiphenyl methane; | 101-77-9 | 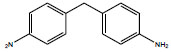 |
Carc. 1B Muta. 2 Skin Sens. 1 |
H350 H341 H317 |
1.59 | >98% | |
| Dicyclohexylmethane-4,4′-diisocyanate | 4,4′-DMDI; 4,4′-HMDI; Methylene bis(4-cyclohexylisocyanate); 4,4'-Methylenedicyclohexyl diisocyanate; Hydrogenated MDI; | 5124-30-1 | 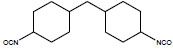 |
Resp. Sens. 1 Skin Sens. 1 |
H334 H317 |
Resp. Sens. 1; H334: C ≥ 0.5% Skin Sens. 1; H317: C ≥ 0.5% |
6.11 | 91% |
| Dicyclohexylmethane-4,4'-diamine | 4,4′-DMDA; 4,4′-HMDA; 4,4'-Diaminodicyclohexylmethane; 4,4'-Methylenebis(cyclohexylamine), | 1761-71-3 | 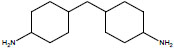 |
Classification not harmonized but notified classification as below** | 3.26 | 95% | ||
| Skin Sens. 1 | H317 | |||||||
| p-Phenylenediamine | PPD; 1,4-diaminobenzene; benzene-1,4-diamine; para-phenylenediamine; | 106-50-3 | 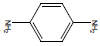 |
Skin Sens. 1 | H317 | 0.43 | 98% | |
| Toluene-2,4-diisocyanate | 2,4-TDI; 4-methyl-m-phenylene diisocyanate; 2,4-diisocyanato-1-methylbenzene; | 584-84-9 | 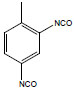 |
Skin Sens. 1 Resp. Sens. 1 Carc. 2 |
H317 H334 H351 |
Resp. Sens. 1; H334: C ≥ 0.1% |
3.74 | 95% |
2. MATERIALS AND METHODS
2.1. Chemicals
4,4′-MDI, 2,4-TDI, 4,4′-DMDI, PPD, and 4,4′-DMDA were obtained from Sigma-Aldrich Chemie GmbH (Steinheim, Germany) and 4,4′-MDA, which was obtained from TCI Europe N.V. (Zwijrdecht, Belgium). Vehicles were acetone of analytical grade obtained from Scharlau Chemie S.A. (Sentemenat, Spain), ethanol from Kemetyl AB (Haninge, Sweden), liquid paraffin from Apoteksbolaget (Stockholm, Sweden), and propylene glycol from VWR International S.A.S. (Fontenay-sous-Bois, France). Sodium lauryl sulphate (SLS) and N,N-dimethylacetamide 99% were bought from Sigma-Aldrich Co (St. Louis, MO, USA), 2-methylol phenol (2-MP) 97% from Acros Organics (Geel, Belgium), and Imject® Freund’s complete adjuvant (FCA) from Thermo Scientific (Rockford, IL, USA).
2.2. Materials
The used materials for the study were the following; Comprilan® 6 cm, elastic compression band was obtained from BSN medical GmbH (Hamburg, Germany), Al-test® from Imeco AB (Södertälje, Sweden), filter papers number 3 from Munktell Filter AB (Grycksbo, Sweden), and 1 ml syringes with injection needle 0.4×20 mm from Codan Triplus AB (Kungsbacka, Sweden). Adhesives bandages were purchased from Durapore™ 3M Health Care (St. Paul, MN, USA) and the plastic adhesive tape from Acrylastic, Biersdorf AG (Hamburg, Germany)
2.3. Ethics
The study was approved by the Lund ethical committee on animal experiments, Lund, Sweden, and conducted in accordance with ethical standards (approval No. M 340-12).
2.4. Guinea-Pig Maximization Test
The GPMT was performed according to the original description [15-17], which is also the method described in the OECD test guideline 406 that can be used to classify skin sensitizers according to the Globally Harmonized System of Classification and Labelling of Chemicals (GHS) [18]. Some modifications of the original method were made, e.g. statistical calculations were used to evaluate potency, furthermore non-irritant epidermal induction concentrations were used and blind readings and a positive control group was introduced in order to be able to standardize the test and objectify the evaluation of the patch test reactions [19-21]. The background for introducing these modifications is specified elsewhere [12, 22]. In Fig. (1) the GPMT in this study is described in detail.
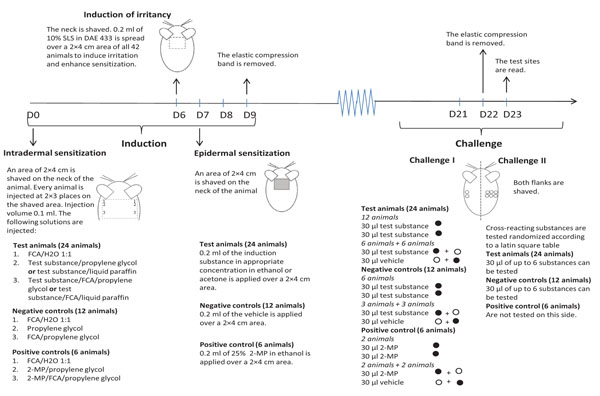
2.5. Animals
Female albino guinea-pigs weighing 400 g (±25 g) of the Hartley-Dunkin strain (HP Lidköpings Kaninfarm, Lidköping, Sweden) were used.
2.6. Topical Irritancy
Before sensitization and cross-reactivity patterns can be assessed, the topical irritancy thresholds have to be determined in order to assure that the chosen test concentrations do not give rise to irritant reactions. This was done by applying different concentrations of each of the investigated substances intended for induction as a closed patch test for 2 days on both the neck and the flank of one side of four animals pre-treated with FCA. In order to maximize the number of test concentrations that could be evaluated the animals were first tested on one side of the body and then on the other side. Concentrations that did not cause irritation were chosen for topical induction and elicitation (Table 2).
| Sensitization Series I | Sensitization Series II | Sensitization Series III | Sensitization Series IV | Sensitization Series V | Sensitization Series VI | |
|---|---|---|---|---|---|---|
|
Induction Intradermal and Epidermal concentrations |
p-phenylenediamine (PPD) 0.43% p.g 0.43% EtOH |
Toluene-2,4-diisocyanate (2,4-TDI) 0.70% p.o 0.70% ac |
Diphenylmethane-4,4′- diisocyanate (4,4′-MDI) 1.0% p.o 1.0% ac |
Diphenylmethane-4,4′-diamine (4,4′-MDA) 0.79 p.g 0.79% EtOH |
Dicyclohexylmethane-4,4′-diisocyanate (4,4′-DMDI) 1.0% p.o 1.0% ac |
Dicyclohexylmethane 4,4′-diamine (4,4′-DMDA) 0.84 p.g 0.84% EtOH |
|
Challenge I Test concentration |
0.43% EtOH C = 1/12 T = 20/24 V = 0/12 Pos = 5/6 p < 0.001 |
0.70% ac C = 2/12 T = 6/16 V = 1/4 Pos = 5/6 p = 0.22* |
** | ** | ** | ** |
|
Challenge II PPD |
0.43% EtOH C = 1/12 T = 18/24 p < 0.001 |
0.43% EtOH C = 3/12 T = 1/16 p = 0.20 |
0.43% EtOH C = 1/12 T = 1/24 p > 0.3 |
0.43% EtOH C = 1/12 T = 2/24 p > 0.3 |
0.43% EtOH C = 1/12 T = 0/24 p > 0.3 |
0.43% EtOH C = 1/12 T = 0/24 p > 0.3 |
| 2,4-TDI | 0.70% ac C = 0/12 T = 1/24 p > 0.3 |
0.70% ac C = 2/12 T = 6/16 p = 0.22 |
0.70% ac C = 1/12 T = 2/24 p > 0.3 |
0.70% ac C = 0/12 T = 3/24 p = 0.28 |
0.70% ac C = 4/12 T = 1/24 p > 0.3 |
0.70% ac C = 1/12 T = 0/24 p > 0.3 |
| 4,4′-MDI | 1.0% ac C = 2/12 T = 2/24 p > 0.3 |
1.0% ac C = 1/12 T = 2/16 p > 0.3 |
** | ** | ** | ** |
| 4,4′-MDA | 0.79% EtOH C = 3/12 T = 21/24 p < 0.001 |
0.79% EtOH C = 5/12 T = 6/16 p > 0.3 |
** | ** | ** | ** |
| 4,4′-DMDI | 1.0% ac C = 0/12 T = 2/24 p > 0.3 |
1.0% ac C = 3/12 T = 8/16 p = 0.17 |
** | ** | ** | ** |
| 4,4′-DMDA | 0.84% EtOH C = 0/12 T = 6/24 p = 0.070 |
0.84% EtOH C = 3/12 T = 6/16 p > 0.3 |
** | ** | ** | ** |
C = negative control animals (in total 12); T = test animals (in total 24); V = reactions to the vehicle in test animals (in total 12); pos = positive control animals (in total 6)
* = 8 animals sacrificed due to strong toxic reactions. P-value not significant.
** presented elsewhere [13].
2.7. Concentrations
Equimolar concentrations were used for the tested substances. The concentrations used for induction and challenge are given in Table 2.
2.8. Induction
24 test animals, 12 control animals and 6 positive control animals were used for induction for each sensitization series (Table 2).
Day 0: All animals were shaved on the neck and thereafter 3 intradermal injections in a row on each side of the shoulder were given, thus 6 injections in total. For the test animals the following injections were made in duplicate: 1) 0.1 ml of 40% FCA in water (w/v); 2) 0.1 ml of the test substance (w/v) in propylene glycol or liquid paraffin; 3) 0.1 ml of a mixture of the test substance and FCA in propylene glycol or liquid paraffin in which the concentration for test substance was the same as in 2) and the concentration of FCA was the same as in 1). For 1) and 2) the vehicle varied depending upon if the sensitizing substance was an isocyanate or an amine since isocyanates can react with propylene glycol which normally is the vehicle of choice. For sensitization series II, III and V liquid paraffin was used and for sensitization and for series I, IV and VI propylene glycol was used (Table 2). For the control animals, the following injections were made in duplicate: 1) 0.1 ml of 40% FCA in water (w/v); 2) 0.1 ml propylene glycol; 3) 0.1 ml of 40% FCA in propylene glycol (w/v). For the positive control animals, the following injections were made in duplicate: 1) 0.1 ml of 40% FCA in water (w/v); 2) 0.1 ml of 25% 2-MP in propylene glycol (w/v); 3) 0.1 ml of 25% 2-MP and 40% FCA in water (w/v).
Day 6: All animals were shaved on the neck and thereafter they underwent a pretreatment of the 2×4 cm area intended for topical induction in order to induce irritancy. The area was treated with 0.2 ml of a preparation consisting of 10% SLS (w/v) in dimethyl acetamide/acetone/99.5% ethanol (DAE) 4:3:3 (v/v/v).
Day 7: All animals were shaved on the neck and thereafter epidermal induction was made in the test animals and the positive controls animals by applying 0.2 ml of the sensitizing substance in acetone or ethanol, depending upon the nature of the sensitizing substance on a 2×4 cm piece of filter paper placed on adhesives bandages. The patches were covered with impermeable plastic adhesive tape and held in place by adhesive bandages. The patches were left on for 48 hours. The control animals were patch tested with the vehicle alone but in the same manner as the test animals and the positive controls.
2.9. Challenge
The challenge procedure consists of two parts; challenge I in which the sensitization rate of the test substance used in the induction is assessed and challenge II in which cross-reactivity to other substances is assessed. Challenge I and II are performed at the same time but on different flanks of the animal; challenge I is performed on the left flank and challenge II on the right.
Day 21: All animals were shaved on their left flank and the test animals and control animals were also shaved on their right flank.
Challenge I (left flank, 2 patches) was performed by challenging 12 test animals with the induction substance in acetone or ethanol, depending on whether it was an isocyanate or an amine, on both the cranial and caudal patch. 6 + 6 test animals were challenged with the induction substance on either the cranial or the caudal patch, and the vehicle (acetone or ethanol) alone on the other patch. 6 of the control animals were tested with the induction substance on both patches and 3 + 3 animals were patch tested with the induction substance on either the cranial or the caudal patch, and the vehicle alone on the other patch. 2 of the positive control animals were tested with 2-MP on both the patches and 2+2 animals were patch tested with 2-MP on either the cranial or the caudal patch, and the vehicle alone on the other patch. Al-test® on Durapore™ adhesive band was used for patch testing. 30 µl test solution was applied. The patches were covered with impermeable plastic adhesive tape and held in place by adhesive bandages.
Challenge II (right flank, 6 patches) was performed on 24 test animals and 12 negative control animals by patch testing with putative cross-reacting substances. The distribution of the positions of the test substance was based on a Latin square table. In this article, cross-reactions to 4,4′-MDI, 4,4′-MDA, 4,4′-DMDI, 4,4′-DMDA in animals sensitized to PPD and 2,4-TDI, respectively, are presented. The results of the investigation of sensitizing capacity of PPD and 2,4-TDI are also presented. The sensitizing capacities of 4,4′-MDI, 4,4′-MDA, 4,4′-DMDI and 4,4′-DMDA as well as the cross-reactivity between the four substances are described elsewhere [12].
2.10. Evaluation
Day 23: The minimum criterion for a positive reaction was a confluent erythema. All tests were evaluated blindly 24 hours after the patch tests had been removed, i.e. 48 hours after test application. First, the left flanks of all the animals were read and thereafter, still blindly and without knowing the test outcome of the left side, the right flanks were read on all animals except the positive controls.
2.11. Statistics
The proportion of positive animals within the test group was compared to the proportion of positive animals in the control group. Among the animals challenged with the induction substance on both the cranial and caudal patches (12 test animals and 6 negative control animals) only one of the patches, chosen in advance, was included.
Statistical significance for the sensitizing capacity and cross-reactivity was calculated with one-sided Fisher’s exact test. When significant values (p< 0.05) were obtained with Fisher’s exact test the compound was considered a sensitizer or showing cross-reactivity to other compounds based upon set criterion (p < 0.001 strong, p < 0.01 moderate, p < 0.05 weak). Indicated cross-reactivity was defined 0.050 ≤ p < 0.3.
3. RESULTS
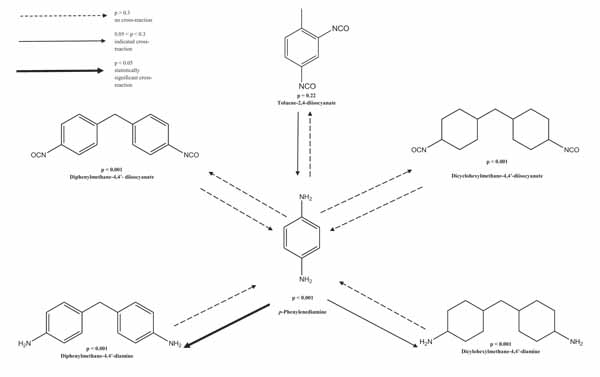
Six different sensitization series were performed at different occasions during a period stretching from May 2013 to September 2015. The results regarding sensitization to PPD and 2,4-TDI as well as the cross-reactivity patterns for each of these series are given in Table 2. For all the sensitization series equimolar concentrations were tested. In Fig. (2), the sensitizing capacity as well as cross-reactivity patterns for the sensitization series are presented.
3.1. Sensitizing Capacity
PPD was shown to be a strong sensitizer (p<0.001). The sensitizing capacity of 2,4-TDI (p=0.22) was based on a test group of 16 test animals instead of 24 since 8 test animals had to be sacrificed due to oozing wounds at the intradermal injection site whose capacity to dry and form crusts was considered to deviate from what was stated in the ethical approval (Table 2).
3.2. Cross-Reactivity
The cross-reaction patterns between the investigated substances when tested equimolar are presented in Fig. (2). Animals sensitized to PPD showed cross-reactivity to 4,4′-MDA (p<0.001). The cross-reactivity did not go in both directions, i.e. animals sensitized to 4,4′-MDA did not show cross-reactivity to PPD. Since 8 animals in the 2,4-TDI induction series (series II, Table 2) were sacrificed statistically significant cross-reactivity patterns could not be fully evaluated.
4. DISCUSSION
4.1. Sensitizing Capacity
The exposure to diisocyanates is usually highest in settings where PUR products are produced industrially. However, exposure in do-it-yourself settings has also been reported [23-25, 26]. The main exposure route for isocyanates is through inhalation [27], and can also occur when PUR products are heated at high temperatures as this leads to degradation of PUR resulting in release of isocyanates, amines and aminoisocyanates [28]. Dermal exposure has been suggested to contribute to respiratory problems and in animal models it has been shown that dermal sensitization could trigger respiratory response when isocyanates are inhaled [29-32].
Contact allergy to isocyanates has been considered a minor problem compared to the respiratory issues and, additionally, much less reported in literature. It has been suggested that the strict rules of handling of isocyanates to avoid respiratory problems have contributed to minimize contamination of skin and thus contact allergy [33]. However, isocyanates do cause contact allergy and there are especially reports describing contact allergy to 4,4′-MDI and 4,4′-DMDI. Many of these reports have also shown simultaneous positive reactions to the corresponding amines, 4,4′-MDA and 4,4′-DMDA [13, 34-39]. In a recent study it was shown that all 4 are sensitizers when investigated with the GPMT [12].
2,4-TDI has been shown to be a strong sensitizer when investigated with the local lymph node assay (LLNA) [40], and has also been described to cause active sensitization in humans following patch testing with 1% 2,4-TDI in pet [40, 41]. Additionally, TDI has been found to be a strong sensitizer both in mouse ear swelling test (MEST) [42] and in the Buehler test on guinea-pigs [43]. LLNA, MEST and Buehler tests are methods that do not involve intradermal injections of allergens as done in the GPMT, in which the allergens are injected intradermally to induce sensitization. In the present study, it could not be statistically significantly shown that 2,4-TDI is a sensitizer based upon our set criteria, when using Fisher’s exact test on the results from the remaining 16 animals. Therefore,it was not possible to fully evaluate the substance since 8 animals were sacrificed due to oozing wounds at the site of the intradermal injection and whose capacity to dry and form crusts was considered to deviate from what was stated in the ethical approval. However, if the sacrificed animals had been positive, the results of 2,4-TDI would have been statistically significant and would have indicated that 2,4-TDI is a moderate skin sensitizer. Notably, based upon the 16 animals that could be read (Table 2) 2,4-TDI fulfil the criteria for classification as subcategory 1B skin sensitizer according to GHS and the CLP regulation since ≥30% to ≤ 60% of the test animals responded at > 0.1% to
In the present study the sensitizing capacity of PPD was also studied. PPD is an ingredient in hair dyes and is considered a potent contact sensitizer. It is usually used to detect hair dye allergy [38, 39, 44]. In this study it was shown to be a strong sensitizer (p < 0.001). This is in accordance with other studies in which PPD has been shown to be a strong sensitizer in both LLNA and GPMT [46-48]. According to GHS and the CLP regulation PPD can be classified as a subcategory 1A skin sensitizer i. e strong sensitizer, since
4.2. Cross-Reactivity
4,4′-MDA has been suggested to be a marker of 4,4′-MDI allergy [9, 10] which was supported when investigating their cross-reactivity pattern with GPMT [12]. In this context, the term cross-reactivity refers to when an individual initially sensitized to one chemically defined substance (A) reacts to a second chemically defined substance (B) that he or she has not been in previous contact with. The first compound is the primary sensitizer while the other is the secondary sensitizer [48]. Cross-reactivity can occur because A and B are structurally similar, or because A is metabolized to a compound that is similar to B and vice versa, or because A and B are both metabolized into similar compounds [49]. Cross-reactivity does not need to go in both directions, i.e if A is a primary sensitizer giving rise to reaction to the secondary sensitizer B it does not automatically mean that a primary sensitization to B also give rise to a reaction to A.
Studies have also reported concurrent reactions between 4,4′-MDA and PPD [13]. One study showed that one third of the 4,4′-MDA positive patients also reacted to PPD [14]. A study presenting clinical patch-test data indicated that “para-amino” compounds could cross-react with each other. Patients positive to PPD were also positive to 4,4′-MDA and other para-amino compounds that are similar in structure [50]. Cross-reactivity has also been reported between PPD and azo dyes [51]. In the present study guinea pigs sensitized to PPD, showed cross-reactivity to 4,4′-MDA which indicates that 4,4′-MDA can be used for detection of PPD contact allergy which supports earlier findings [13]. The cross-reactivity between 4,4′-MDA and PPD should be taken into consideration if 4,4′-MDA is used as a marker for 4,4′-MDI and it should be considered that a positive reaction to 4,4′-MDA could also be a sign of hair dye habits and not only isocyanate exposure. Since no cross-reactivity could be found between 4,4′-MDI and PPD, one can assume that, individuals with hair dye allergy can work with isocyanates. However, it is noteworthy that in many plastic applications, such as PUR production and epoxy applications, 4,4′-MDA at least has been used as a hardener. Thus, nothing in the results from this study suggests that 4,4′-MDI sensitized individuals have a higher risk to develop eczema when dying their hair. However, we cannot draw any conclusions on whether 2,4-TDI sensitized individuals are more likely to develop eczema from hair dyes containing PPD since the result could not be fully evaluated due to the sacrifice of 8 animals which resulted in non-significant p-value. However, it should be noted that an indicated cross-reactivity to PPD was seen in animals sensitized with 2,4-TDI (p=0.20). It is possible that significant results had been seen if all the sacrificed animals had been positive. In a study by Tanaka et al, cross-reactivity between MDI and 2,4-TDI was indeed shown in MEST [52].
In the present study there was also an indicated cross-reactivity to 4,4′-DMDA (p=0.069) in animals sensitized to PPD. In this study all substances were tested equimolar to each other in order to be able to compare the sensitizing capacities between the investigated substances. However, the GPMT is a method that is defined by maximization which, according to the original method, means that the animals are sensitized with the highest non-irritating concentration of the test substance regardless of the equimolarity. If the substances in this study had been tested according to the original method it is possible that statistically significant numbers of reactions had been seen for 4,4′-DMDA in the animals sensitized to PPD. This, together with the fact that the previous GPMT-study [12] showed cross-reactivity to 4,4′-DMDA in animals sensitized to 4,4′-MDA and an indicated cross-reactivity in the reversed situation highlights the need of further studies to investigate the cross-reactivity patterns between the investigated amines and other structurally close substances such as 2,4-TDA, Disperse Orange 3 and other azo-dyes.
CONCLUSION
PPD was shown to be a strong sensitizer. PPD-sensitized animals showed cross-reactivity to 4,4′-MDA. However, PPD cannot be used as a marker for isocyanate contact allergy. Our results indicate that allergy to 4,4′-MDA can indicate sensitization to either PPD or 4,4′-MDI or to both. Our results do not support the suspicion that PPD allergic individuals should avoid working with isocyanates since no cross-reactivity between PPD and 4,4′- MDI could be shown. The intradermal induction concentration of 2,4-TDI (0.70% w/v) can induce strong local reactions in guinea-pigs and should be lowered.
AUTHOR CONTRIBUTIONS
All authors have participated sufficiently to take public responsibility for the work.
FUNDING
Swedish Research Council for Health, Working Life and Welfare.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the Lund ethical committee on animal experiments, Lund, Sweden, and conducted in accordance with ethical standards (approval No. M 340-12).
HUMAN AND ANIMAL RIGHTS
The work is approved by the swedish/european ethical comitee of animal testing.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors thank Monica Andersson, Lena Persson and Lotta Thorsson for skilful technical assistance and the Swedish Research Council for Health, Working Life and Welfare for providing funds.


