All published articles of this journal are available on ScienceDirect.
Possible Spironolactone Induced Intracranial Hypertension in a Patient with Androgenetic Alopecia: A Case Report
Abstract
Spironolactone is a well-known drug with many indications. In dermatology, it may be used for treating androgenetic alopecia with a high androgen level. A patient with idiopathic papilledema that was inactive for many years experienced a significant increase in intracranial pressure after receiving spironolactone. The symptoms were resolved soon after the medication was discontinued. This report draws physicians’ attention to such rare adverse events that may have unwanted consequences.
1. INTRODUCTION
Female androgenetic alopecia (AGA) is a well-known hair disorder that mostly affects middle-aged women [1]. Several pharmacological treatments for AGA are available, including spironolactone which displays an antiandrogenic effect. However, spironolactone-containing drugs are associated with many side effects, including orthostatic hypotension, electrolyte imbalance, menstruation irregularity, breast tenderness, hematological disorders, urticaria and fatigue [2]. An increase in the intracranial pressure is rare, but may occur after consuming spironolactone. Here, we report such an adverse effect of spironolactone along with literature review.
2. CASE PRESENTATION
This case includes a 39-year-old woman with class 1 obesity and polycystic ovarian syndrome (PCOS), which significantly exacerbated her hair loss. She also had a history of idiopathic intracranial hypertension (IIH) that was diagnosed at 14 years of age and treated with steroids and diuretics. The treatment has been discontinued since 13 years. The patient’s medications included levothyroxine (100 µg daily) for hypothyroidism and atorvastatin (10 mg) for hypercholesterolemia. She visited the dermatology clinic complaining of hair loss since 1 year, and was diagnosed with Ludwig stage 1 female pattern hair loss. Daily topical solution of minoxidil and oral solution of spironolactone (50 mg) were prescribed. After 3 months, the patient reported new facial hair growth. No adverse effects were observed; thus, spironolactone dose was increased to 100 mg daily. Two months later, the patient presented at the neurology clinic due to visual obscuration, blurred vision, dizziness, pulsating tinnitus, and headache. These complaints were observed since the beginning of the treatment and were mild, but then progressively worsened with treatment maintained up until presentation at the neurology clinic. The initial impression was recurrence of IIH and the patient was advised for further evaluation. She refused to undergo lumbar puncture. Magnetic resonance imaging (Fig. 1) and magnetic resonance venography (Fig. 2) were performed, and acetazolamide (250 mg) was prescribed. Ophthalmologic examination revealed an optic nerve head showing clear temporal margins and evidence of old blurring of the superionasal margins. Venous pulsation was evident; there were no overt signs of acute papilledema. The final impression was recurrence of bilateral papilledema. The patient continued to complain of dizziness, frontal headache, which was throbbing in character, and associated with occasional nausea. She also reported a weight gain of 6 kg in 1 week, without any changes in diet and exercise. Spironolactone administration was discontinued, but minoxidil application was continued. After six weeks, all the symptoms were significantly improved. Then, acetazolamide administration was also discontinued. There were no electrolyte disturbances or blood pressure abnormalities detected throughout the spironolactone treatment.
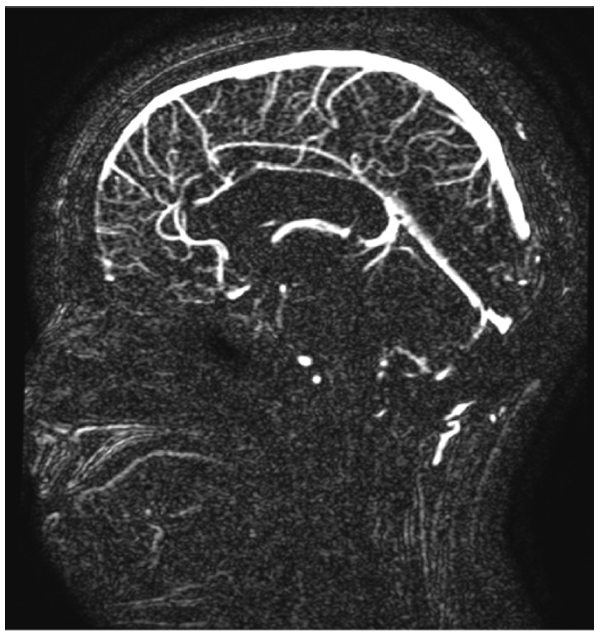
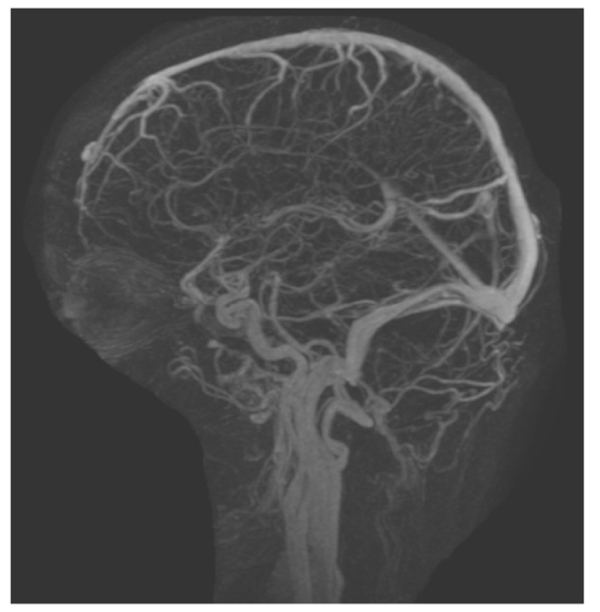
3. DISCUSSION
Spironolactone is an androgen-dependent drug. It displays an antiandrogenic effect due to competitive blocking of androgen receptors, inhibition of ovarian androgen production, and aldosterone antagonism. Its side effects include orthostatic hypotension and electrolyte imbalance. Therefore, blood pressure and electrolyte evaluation should be routinely performed during the treatment course [2].
We report this case with a possible rare side effect of spironolactone in a patient treated for female pattern hair loss due to unknown mechanism and with chronic IIH. Many diseases have been linked with IIH, such as hypothyroidism [3], and PCOS [4], as observed in our patient.
Our patient presented in the neurology clinic with classical IIH symptoms, and after evaluation, the initial impression of recurrence of IIH was confirmed. Spironolactone was discontinued, but minoxidil was continued. Six weeks later, all her symptoms significantly improved, and treatment with acetazolamide was then discontinued. The patient’s course indicates a plausible association of recurrence/worsening of IIH with spironolactone, which is also supported by a Naranjo score of 3, which rates the likelihood of this particular case of adverse drug reaction as “possible” [5]. The underlying pathophysiology in this case remains unknown. However, we speculate that spironolactone affects the central and peripheral neurotransmitter system by deregulating the dopaminergic and γ-aminobutyric acid activity [6] in a patient who is prone to IIH. Although spironolactone is known to cause hyperkalemia and blood pressure abnormalities [7], these abnormalities were not observed in our patient. A literature review did not reveal any similar cases. However, spironolactone was reported to have caused dizziness, which may be related to hypotension, in a series of 20 women with hidradenitis suppurativa [8]. Spironolactone has been used in the treatment of female pattern baldness [3], and it acts by decreasing the levels of 17b-hydroxylase and desmolase, which are androgen synthesis-dependent enzymes [9]. In the treatment of hypertension, it acts as a mineralocorticoid receptor antagonist [9]. Currently, spironolactone has been reformed to be administered topically to reduce its systemic side effects with the use of nanomedicine properties [10].
CONCLUSION
Dermatologists should be aware of this rare side effect. We recommend that spironolactone should be used with caution in patients with chronic IIH, and that patients should be informed about the nature of its adverse symptoms.
ETHICAL APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by Ethical commission of King Saud University.
HUMAN AND ANIMAL RIGHTS
No Animals were used in this research. All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2008.
CONSENT FOR PUBLICATION
A written informed consent was obtained from the patient.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


