All published articles of this journal are available on ScienceDirect.
Disinfectants and Skin Antiseptics for Safe prophylaxis against COVID-19, Review of Literature.
Abstract
Coronavirus disease 2019 (COVID-19) is currently receiving the whole world's attention. It appeared first in Wuhan city of China and rapidly spread to the world, causing many mortalities and morbidities; the disease is mainly transmitted via respiratory droplets and has a long infectivity period of about 14 days.
Science shows that the virus is also transmitted via the skin if the virus by any means finds its way and land on the skin surface. Infection occurs when touching the face, eyes, or nose with the hand after the virus has landed upon it. This is the main reason for the widespread usage of skin antiseptics and disinfectants. We included the most commonly used skin antiseptics, sterilizing methods, and disinfectants, such as household bleach, hydrogen peroxide gas plasma, Formaldehyde, Glutaraldehyde, Alcohol, Chlorohexidine, Povidone-iodine, Chloroxylenol, and alcohol-based hand sanitizer (e.g. Sterlelium). We will discuss their role in preventing acquired infection of COVID-19, as well as discussing the efficacy, costs, and side effects of different sterilizers, including general health hazards, as well as skin affection as irritant contact dermatitis, which is the commonest side effect. After conducting this work, we summarized the results & started sending them to our patients & medical personnel, and we observed 60% decrease in the cases of disinfectants induced allergic contact dermatitis /month compared to the previous two months.
1. INTRODUCTION
Coronavirus disease 2019 (COVID-19) is a recent pandemic throughout many countries, triggered by the novel coronavirus (2019-nCoV). The virus is characterized by a long incubation interval and a virulent infectivity rate, which has served in increasing mortalities to over thirty-three thousand people by the end of March 2020 [1]. As soon as the virus caused infection for more than 100 thousand people in more than 100 countries, the World Health Organization (WHO) has considered COVID-19 as a pandemic [2]. The virus has affected more than a million people and leads to many causalities across various countries [3]. Subsequent studies and research showed that older patients, especially diabetic and hypertensive patients, are the most susceptible age group with the highest case fatality rates [4]. The causative organism is an enveloped virus of the Coronavirus family, characterized by many glycoproteins of spikes protruding from the exterior of the virus, which gives the picture of a “corona” and hence the nomenclature [5].
Science has now revealed the quaternary structure of the S-glycoprotein that is found on the surface of the virus “S is derived from Spike” [6]. It can be easily obtained now from the Protein Data Bank (PDB). The S glycoprotein is responsible for the virulence of the virus and plays a critical role in the infectivity of human target cells, through the cell-to-cell attachment of viral membranes with human cell membranes, this leads to the fusion of both membranes and allows the viral contents and genetic material to pass to the nucleus where replication starts [7]. The enveloped nature of the virus renders it vulnerable to many detergents and susceptible to various disinfectants and antiseptics (Fig. 1) [8].
In this review, we aim to collect and address the efficacy of different disinfectants and skin antiseptics as prophylactic measures against the COVID-19. Disinfectants are chemical substances that can be applied only to inanimate objects. They are effective against variant types of pathogenic organisms, including the lipophilic, enveloped, viruses such as influenza viruses and coronaviruses. They neutralize the infection caused by these viruses by destroying the envelope coat that helps them attach to the host cell receptor [9]. This review was collected by reviewing all related papers from internet sources (google scholar, Medline, PubMed and Medscape).

2. HOUSEHOLD BLEACH
Sodium hypochlorite is classified as one of the furthermost extensively used chlorine disinfectants, and it is the utmost predominant chlorine product in the United States [10]. Sodium hypochlorite (NaOCl), also called household bleach, is made by diluting the laboratory bleach. It is a broad-spectrum antimicrobial agent killing bacteria, fungi, and viruses, such as influenza virus, it needs about 60 minutes to achieve the mission of disinfection [11, 12]. The exact mechanism by which Sodium hypochlorite solutions exhibit their antimicrobial action is still unknown, however, free chlorine may cause oxidative damage to sulfhydryl enzymes in the cell, damage of these enzymes inhibits protein synthesis, reduces oxygen uptake, destroys DNA and causes cell death [12]. Sodium hypochlorite is widely used and obtainable at a minimal cost. Therefore, it is highly proposed for surface disinfection in healthcare facilities. However, bleach-like other disinfectants can irritate the skin and mucus membranes.
Sodium hypochlorite solutions are unstable, under the effect of heat and air, chlorine evaporates, reducing the level of free chlorine in these solutions, which decreases their antimicrobial action. The following precautions should be kept in our minds when we deal with any Sodium hypochlorite solutions: Take care not to touch your eyes. If the bleaching agent reaches the eyes, wash them with running water and see a physician. Keep them away from metal and painted surfaces to protect them from its corrosive action. Do not use them together with other chemical detergents since this may lead to dangerous chemical reactions.
For example, mixing acidic detergents with bleach produces toxic gas that can cause serious injury and death. Organic materials inactivate bleach. Therefore, clean surfaces from any organic materials before adding bleach [11].
3. QUATERNARY AMMONIUM COMPOUNDS
Benzalkonium chlorides (BACs) are commonly used disinfectants, also recognized as ammonium alkyl dimethyl (phenylmethyl) chlorides, alkyl dimethyl (phenylmethyl) quaternary ammonium chlorides or alkyl dimethyl benzyl ammonium chlorides; they are named according to the lengths of the alkyl chain forming these compounds [13]. The first BACs containing product was registered with the EPA in 1947 [14]. Since that time, they have been widely used in different products. The antimicrobial action of the quaternary comes from their capability to disable the energy-producing enzymes, denature essential cell proteins, and interrupt the cell membrane [15, 16]. Many published scientific literatures showed that quaternary disinfectants are generally effective virucidal against lipophilic (enveloped) viruses, including respiratory tract viruses [17, 18]. However, nosocomial infections have been stated from contaminated quaternary ammonium compounds consumed for cystoscopes or cardiac catheters [19, 20].
They are used as a routine disinfectant in our regular environmental hygiene and uncritical surfaces such as floors, furniture, and walls.
4. HYDROGEN PEROXIDE GAS PLASMA
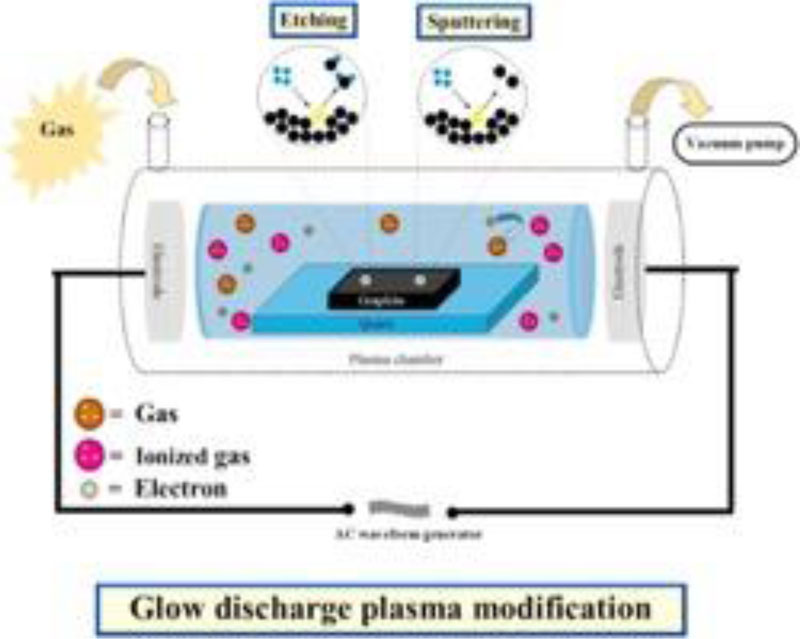
Gas plasmas are produced in a closed cavity under a deep-seated vacuum (Fig. 2) The gas in this chamber is excited using radiofrequency waves to produce hydroxyl free radicals and ionized gas. These hydroxyl free radicals attack cell constitutes such as cell membranes, DNA, and essential 1 14 2 15 proteins of the cell. Unfortunately, catalase generated by aerobic and facultative anaerobes organisms can protect these cells by degrading hydrogen peroxide to oxygen and water [3]. Hydrogen peroxide is effective against an extensive range of microbes. It needs about one minute to achieve its viricidal activity [21].
5. FORMALDEHYDE
Formaldehyde is used in both liquid and gas forms as a disinfectant and sterilant. Formalin is a water-based solution, and it is about 37% formaldehyde when it comes to weight [4]. This aqueous solution is highly tuberculocidal, bactericidal, sporicidal, viricidal and fungicidal. Formaldehyde destroys the microbes by alkylating the sulfhydryl and amino groups of essential protein [17, 18]. Formaldehyde is potentially carcinogenic. Therefore, exposure of the employee to formaldehyde should be limited to an average exposure concentration of 0.75 ppm for an eight-hour weighted time [5].
6. GLUTARALDEHYDE
Glutaraldehyde, C5H8O2 or OCH(CH?) CHO, is a liquid substance with a very powerful scent. It is a toxic chemical ingredient used as a sterilant to clean Medical and surgical equipment. In addition to that, glutaraldehyde is also used in tissue fixative in labs and hardening in X-ray evolution. Glutaraldehyde has a role as a biocide in fluids, oil, and gas. Glutaraldehyde's alkylation of sulfhydryl, carboxyl, hydroxyl, and amino groups of microorganisms results in its antimicrobial effect, which alters DNA, RNA, and protein synthesis [6].
Glutaraldehyde does not cause corrosion of metals. It does not damage the lens system, whether in rubber or plastic hardware. Another advantage of this article is that it is considered fairly cheap. Many studies give us instructions for dealing with glutaraldehyde. Glutaraldehyde irritates the respiratory system causing shortness of breath, sneezing, and cough. The level of risk depends on the level and form of exposure to this substance.
7. ALCOHOL
Price [22]collected and illustrated various studies in the late 1800s that investigated the effectiveness of using alcohol as a disinfectant or antiseptic. Studies revealed that, during testing of alcohol concentrations starting from 25% towards 99%, 50% was found to be the most germicidal. On testing the efficacy of 15% to 99% alcohol concentration on silk threads with numerous types of bacterial families, it has been shown that 50% concentration exerts the most efficacy on gram-positive organisms, especially staphylococcus aureus. The furthermost acceptable clarification for the antimicrobial effect of alcohol is the proteins' denaturation. Alcohols have excellent activity against gram-positive bacteria, gram-negative bacteria, enveloped viruses, non-enveloped viruses, mycobacteria, and even fungi.
This process is reinforced by the inspection that pure ethyl alcohol, a dehydrating agent, is to a lesser extent bactericidal than combinations of alcohol and water since proteins are denatured further rapidly in the existence of water. Since alcohol is combustible, to be used as a surface disinfectant is limited to a small surface area and in well-ventilated spaces only. Long term use of alcohol as a disinfectant can also cause discoloration, and cracking of rubber [11].
8. ANTISEPTICS
8.1. Chlorohexidine
Chlorohexidine is considered a sterilizer and antiseptic that is consumed before surgery for skin decontamination and to sanitize surgical instruments [23]. It may be beneficial to decontaminate the skin of the healthcare providers and the patient [24]. It is on the World Health Organization's Listing of Essential Medicines, the securest and highest efficient medicines required in a health structure [25]. The bactericidal impact is an outcome of the binding of this cationic particle to negatively-charged bacterial cell walls. At decreased concentrations of chlorhexidine, this causes a bacteriostatic upshot; at elevated concentrations, membrane disruption develops cell death [26]. Additionally, it is ineffective in opposition to adenoviruses and polioviruses. The efficiency of chlorhexidine towards herpes viruses has not yet been determined [27].
Side effects may contain teeth discoloration, allergic reactions, and skin irritation, and if close contact happens, it may also initiate eye problems. Chlorhexidine may be in a mixture with a surfactant solution, alcohol, or water. It does not deactivate spores; however, it is effective against a diversity of microorganisms [27, 28]. Chlorohexidine does not follow the current European specifications for the sanitizer of the hand. Under the trial settings of the European Standard EN 1499, no considerable difference in the effectiveness was found amongst a 4% solution of chlorhexidine gluconate and soap [27, 28]. A Medical Center, in the U.S., between 2007 and 2009, performed a cluster-randomized trial and deduced that patients daily bathing in intensive care units with chlorhexidine gluconate diminished the risk of hospital-acquired infections [27, 28]. It still unclear if long-standing exposure for a lot of years may have carcinogenic potential. The Federal Drug Administration (FDA) in the USA advises minimizing its mouthwash use to not more than six months [29].
CHG is scantily absorbed across the gastrointestinal tract Once digested [30, 31].
While it can be fatal due to the high risk of acute respiratory distress syndrome, as reported in one case, once aspirated into the lungs at high enough concentration [31].
8.2. Povidone-iodine
Povidone-iodine (PVP-I) is an antibacterial and disinfectant, benefitted before and after surgery for disinfection of the skin [24, 25]. It may be utilized to disinfect the hands of healthcare personnel. It may also be consumed for minor wounds [24]. It may be used as an application to the skin, either as a powder or a liquid [24]. Povidone-iodine was used for commercial purposes in 1955. It is one of the Essential Medicines on the World Health Organization's List, the most harmless and most valuable medicines demanded in a health system. It is available as an over-the-counter product [32]. The comprehensive cost is in the range of US$3.30 to US$11.40 for a liter of 10% solution in the developing world. This quantity in the United Kingdom charges the NHS about £10.86. 28 It is advertised under numerous brand names like Betadine [27, 28].
Its mechanism involves releasing iodine, which causes the death of a range of microorganisms [29]. It can be used as a broad-spectrum antibacterial for a local appliance in the management and avoidance of wound infection. It may be used as a part of the first aid for grazes, minor cuts, burns, blisters, and abrasions. Povidone-iodine reveals longer-lasting disinfected effects than the tincture of iodine, due to its gradual absorption in soft tissue, causing it to be the product of choice for prolonged surgeries. Chlorhexidine has the same outcomes but has the same toxicity worries [32].
Subsequently, PVP-I has obtained extensive application in the medical field as surgical scrubbing; for pre- and post-operative skin sanitization decontamination; for the prevention and treatment of infections in wounds, burns, cuts, and ulcers; for the management of infections in stasis ulcers and decubitus ulcers; and for vaginitis in gynecology. For these roles, PVP-I has been articulated at concentrations of 7.5-10.0% in spray, solution, swab dosage forms, surgical scrubbing, and ointment [33, 34]. Side effects contain high blood sodium, kidney problems, metabolic acidosis and skin irritation if used on large wounds. For people who are less than 32-week pregnant or on lithium, it is not suggested.
Recurrent usage is not recommended for people having thyroid problems. Povidone-iodine is a chemical composite of elemental iodine, povidone, and hydrogen iodide. It comprises of 9% to 12% available iodine.
8.3. Chloroxylenol
Chloroxylenol is a disinfectant and antiseptic which is consumed for skin decontamination and scrubbing surgical instruments. It is also applied to several sanitizers and wound cleaners. Still, it is less efficient than some other existing agents [27, 28]. Chloroxylenol was first made in 1927, and it is on the World Health Organization's List of Essential Medicines, the safest and utmost effective medicines required in a health system. The extensive cost is about US$2.00-6.20 per liter of 5% solution in the developing world. It is marketed in several preparations and under a number of brand names, including Dettol [27, 28]. Side effects are mostly scarce but can contain skin irritation. It may be utilized mixed either with alcohol or water. Chloroxylenol is mostly successful against gram-positive bacteria. It works by the interruption of the cell wall and discontinuing the function of enzymes [29-31]. Chloroxylenol is used in households and hospitals for sanitation and disinfection. It is also frequently used in antiseptic soaps, wound-cleansing products and household sanitizers such as Dettol liquid [32-35].
Extreme contact with chloroxylenol has the capability of causing death. If swallowed, it can be toxic and even when it is accidentally inhaled. A medical study in Hong Kong which investigated 177 cases of Dettol digestion that resulted in treatment in the emergency department (95% of which were intended), deduced that “Dettol could be poisoned and cause dangerous complications in 7% of patients, even death [35, 36].
8.4. Hand Sanitizer (Sterillium)
Hand sanitizer is utilized to eliminate infectious agents on the hands. They are available in the form of foams, liquids, and gels. Preparations of alcohol-based forms are better than handwashing, in the healthcare locations, with water and soap. Usually [36, 37], it is more useful than soap and water at eradicating germs. The widespread usage of non-alcohol-based varieties has no endorsements. For an external healthcare setting, using soap and water for handwashing is still preferred [38]. A combination of ethanol (ethyl alcohol), n-propanol, or isopropyl alcohol typically exists in alcohol-based versions, while some versions, containing 60% to 95% alcohol, have the greatest effect.
They have a great effect on a wide scope of microorganisms but not on spores. To protect the skin from drying, Composites like glycerol may be included [39]. They have been frequently consumed in Europe since at least the 1980s. Its version is included in the World Health Organization's Listing of Essential Medicines, the most effective and securest medicines desirable in a health organization. In the developing world, the comprehensive cost is in the range of 1.40-3.70 US$ for one liter [38-40]. Hand sanitizer that includes no less than 60% alcohol, Alcohol rubs eradicate several diverse types of bacteria, involving antibiotic-resistant bacteria, T.B. bacteria and many kinds of viruses, including enveloped viruses, for example, HIV, the flu virus, coronaviruses, and the common cold virus, though it is remarkably useless against the rabies virus [41, 42]. Ninety percent of alcohol rubs are further successful against viruses than most other methods of hand cleaning [42]. Isopropyl alcohol will eradicate 99.99% or more of all non-spore-forming bacteria in less than 30 seconds, both on human skin and in the laboratory [43].
The alcohol in hand sanitizers may not take the 10-15 seconds exposure time essential to lyse cells and denature proteins is too low amounts (0.3 ml) or concentrations (below 60%) in environments containing high protein or lipids waste (such as food processing), using alcohol hand rubs on its own may not be adequate to guarantee the intended hand hygiene [44]. For settings of health care, like clinics and hospitals, the optimal concentration of alcohol needed to eradicate bacteria is 70% to 95%. While in American stores, Products that have alcohol concentrations as low as 40% are accessible, according to researchers in the East Tennessee State University [45]. Rub sanitizers having alcohol could exterminate most of the bacteria and fungi, and may eradicate some viruses. Alcohol rub antiseptics, including a minimum 70% alcohol (mainly ethyl alcohol) could kill 99.9% of the bacteria on hands within 30 seconds after applying and to the extent of 99.99% to 99.999% within one minute [46, 47].
Side effects of hand rubs with alcohol do not establish any risk by eliminating the naturally present beneficial microorganisms on the skin. The beneficial microbes on the hands get quickly replenished by the body, often relocating them in from just up the arms where there are fewer damaging microorganisms [48].
CONCLUSION
Several disinfectants have been proven highly effective against enveloped viruses, such as ethanol, povidone-iodine and bleach (Sodium hypochlorite) [49].
Household bleach is very effective; however, it is irritant on the skin and mucus membranes. In a study in which occupational dermatitis was evaluated in health care workers, the hands are most commonly affected. This most likely occurred because frequent hand washing, gloves, disinfectants, and detergents are known irritants [50].
Its efficacy also decreases over time. Hydrogen peroxide immediately destroys bacterial cell membranes and DNA; however, it is not practical for everyday use and is expensive. Formaldehyde and glutaraldehyde can both be used in the liquid form. However, they are potentially carcinogenic. Alcohol is very effective and suitable for everyday use. Chlorohexidine seems to exert the same efficacy as ordinary soap. Betadine is the most suitable antiseptic solution used in healthcare facilities due to its long-acting period and slow absorption rate by the soft tissue. Dettol has a potent action against gram-positive organisms but has a high cost.
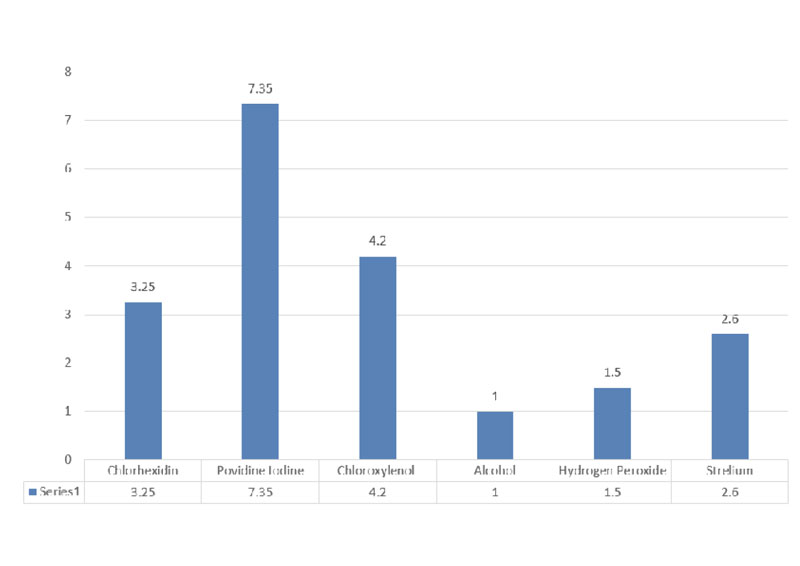
Alcohol-based hand sanitizers are considered the best option regarding both the cost and efficacy, however, their use in an environment with high lipid and protein wastes are poorly effective recommendation: The standard price of each antiseptic, alcohol has the lowest price of all of them (Fig. 3) and the sterillium has the safest. Strelium is an alcohol derivative and safe for skin and not toxic, such as the rest of them. In our literature review, we recommend sterillium as a disinfectant for the skin against COVID-19.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


