All published articles of this journal are available on ScienceDirect.
Dermoscopic Findings in Cases of Cutaneous Metastases
Abstract
Background:
Cutaneous metastases are cancerous cells in the dermis and hypodermis and can develop from every type of malignancy. The involvement of the skin in the metastatic process is considered to be quite rare and carries a poor prognosis, but it is of great importance in the management, treatment and self-esteem of the patient.
Objective:
The objective of this paper is to collect research data on clinical signs of cutaneous metastases and the use of dermoscopy in their diagnostic process.
Results:
Cutaneous metastases present with different clinical variants and dermoscopic findings, the most common being non-painful skin-colored nodules with various vascular structures seen in dermoscopy. There are not many reports on the dermoscopic findings of cutaneous metastases.
Conclusion:
Cutaneous metastases remain a rare diagnosis but are of great clinical importance. As the use of dermoscopy rises yearly, a better understanding of the dermoscopic features in cutaneous metastases should be obtained and reported.
1. INTRODUCTION
Cancer remains a crucial public health issue worldwide. A major problem with the management and treatment of malignancies is their ability to produce metastases.
Cutaneous metastases (CM) are cancerous cells in any layer of the skin, originating from primary cancer [1]. These tumor cells metastasize through haematogenous, lymphatic, and direct tissue invasion. Even some cases of iatrogenic malignant implantation have been reported [2]. Regarding the frequency of skin metastasis formation, it correlates with the frequency of primary cancer [3]. The most common types of cancers to produce CM are melanoma, breast cancer, lung cancer, colon cancer and others, but theoretically, every type of cancer can form metastases in the skin (Table 1) [4]. As 1-10% of all cancer patients with metastatic disease will develop cutaneous metastases, they are quite rare in everyday practice but of great importance. Skin involvement in the metastatic process is considered a poor prognosis for the overall patient condition [5].
| Males | Females |
|---|---|
| Lung cancer (24%) Colon cancer (19%) Melanoma (13%) Squamous carcinoma of the oral cavity (12%) Kidney cancer (6%) Stomach cancer (6%) Oesophageal cancer (3%) |
Breast cancer (69%) Colon cancer (9%) Melanoma (5%) Ovarian cancer (4%) Cervical cancer (2%) |
2. MATERIALS AND METHODS
This review was prepared by performing a comprehensive search of the literature using keywords related to cutaneous metastases and their dermoscopic findings. The search was run on January 2021, in EBSCOhost, ScienceDirect, Wiley Online Library and ClinicalKey databases. Book chapters, case studies, case reports and literature reviews were included.
Inclusion criteria included the articles on the topic of cutaneous metastases formed by internal malignancy, melanoma or lymphoma, and those containing clinical and/or dermoscopic images of the cutaneous metastases. Exclusion criteria involved report on primary skin tumors, non-English studies, and if full articles are not available.
A total of 580 citations were generated from the literature search, of which twenty-two (n=22) met the inclusion criteria. For the analysis of dermoscopic patterns in cutaneous metastases, only papers that provided dermoscopic images were considered. Ten (n=10) of the analysed reports were used to provide a summary of the available data on dermoscopic features in cutaneous metastases. Pictures from the author’s private collection were added for additional visual purposes.
3. RESULTS
3.1. Clinical Features
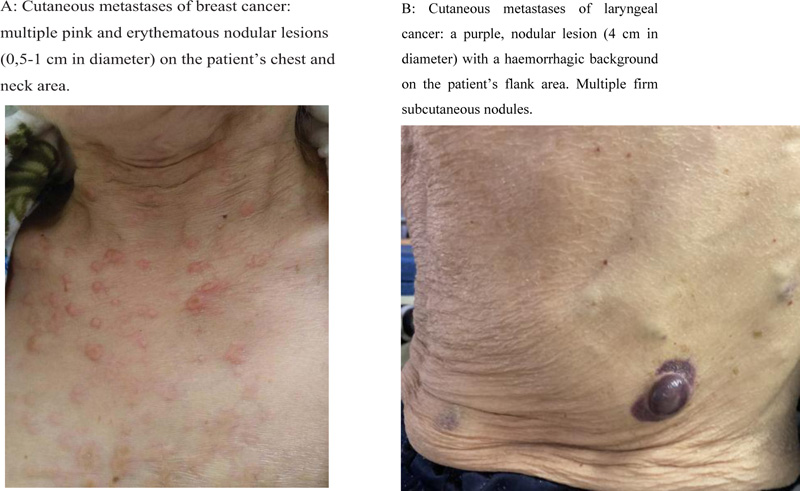
The most common clinical presentation of cutaneous metastases involves painless, firm nodules located in the dermis, anatomically near the primary tumor site, metastatic lymph node or surgical scar [1, 5, 6] (Fig. 1). These nodules usually appear suddenly and show a rapid enlargement. The average size of a nodule is between 1 to 3 cm, but much larger and smaller lesions have been reported [7]. Various other clinical forms of skin metastases have been reported as well. For example, cutaneous metastases can mimic dermatitis [8] and even chancres [9]. The migration of the malignant cells can also cause lymphatic obstruction, presenting as facial swelling and elephantiasis [7].
The color of the newly formed lesion varies from flesh-colored to pink, red, purple, and even black. In breast cancer, such specific forms as induration (peau d’orange), erysipelas-like formations and erythematous papules that resemble vascular proliferation are described. In leukemia and lymphoma patients, papular and nodular pink-to-brown lesions have been described as a form of cutaneous metastases [5, 10-12].
It is also worth mentioning that cutaneous metastases can become infected by various pathogenic and/or opportunistic bacteria (such as S. aureus, P. aeruginosa), causing discomfort, pain, malodor, and other complications [13].
3.2. The Role of Dermoscopy in Diagnostics Of Cutaneous Metastasis
There are no specific steps for acquiring the diagnosis of skin metastasis. The appearance and anatomical site of the newly formed lesion as well as the patient’s history can play an important role in the diagnostic process. Although lesion biopsy is considered the most effective diagnostic method, an additional, non-invasive diagnostic approach would be dermoscopy.
Dermoscopy (or epiluminescence microscopy, ELM) is a widely used method in clinical practice to mainly inspect benign and malignant nevomelanocytic lesions. A study carried out in 2017 by Christoph Sinz et al. suggests that dermoscopy also improves the diagnosis and management of non-pigmented skin cancers and should be used as an adjunct method to the basic examination of suspicious lesions [14].
The available information on the dermoscopic patterns of cutaneous metastasis is limited; we found ten publications on this topic (Table 2).

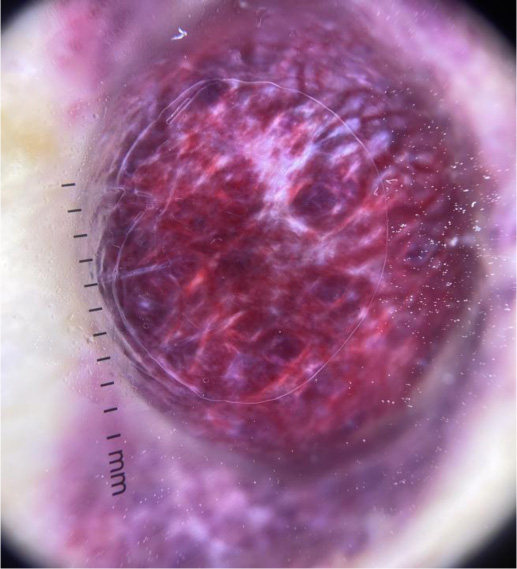
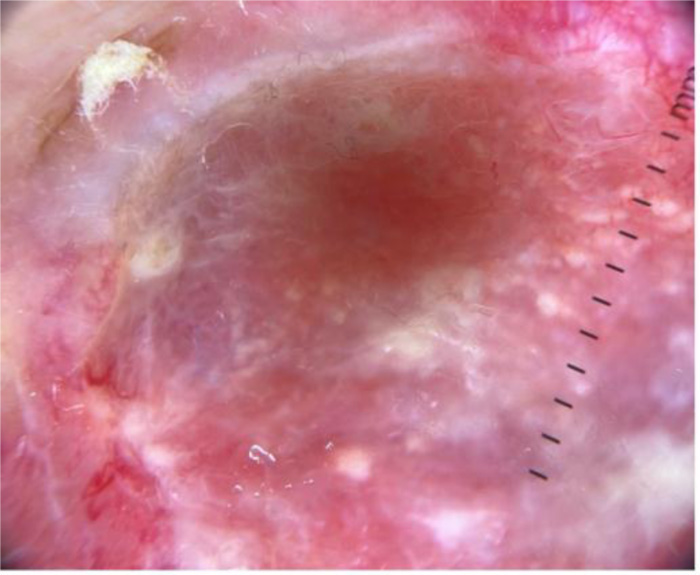
Karen A. Chernoff et al., in their case series, reported a high prevalence of vasculature in at least 88% of cases with serpentine, arborized, dotted and comma shaped vessels, sometimes giving a mixed pattern. Some breast cancer cutaneous lesions in the study presented with clinical hyperpigmentation with pigmented streaks or globules in dermoscopy [1]. Another case study of breast cancer by Awatef Kelati and Salim Gallouj reported linear irregular and polymorphic vessels combined with white bright lines and white structureless areas [15].
In a case report of cutaneous metastasis of renal carcinoma, dermoscopy revealed purple-red colour lesions with multiple linear vessels that were distributed in a parallel pattern in the centre of the lesion. White lines in the periphery of the lesion were also described [16].
In reports on cutaneous melanoma metastases, the main dermoscopic patterns reported are homogenous, saccular, amelanotic, vascular and polymorphic [17, 18]. Rubegni et al. suggest that the vascular patterns are related to tumor thickness; corkscrew vessels are more often found in thick lesions while punctate vessels predominate in thinner ones. Vascular structures are more often found in melanoma metastases than in primary lesions, thus they could be a valid diagnostic tool for distinguishing primary melanoma from its metastases [18]. Vascular patterns (serpentine, hairpin, and other types of vessels) are also reported by Jaimes et al. in non-pigmented melanoma metastases. Other dermoscopic patterns include peripheral grey spots/globules, pigmentary halo, and perilesional erythema [17-19].
The main dermoscopic findings in cutaneous forms of lymphomas include various presentations of vessels (ex., linear, dotted, arborizing), structureless areas and yellow areas, as well as scaling [12].
Some examples of dermoscopic images from cutaneous metastases are presented in (Figs. 2-4).
| Author(s), Year of Publication |
Publication’s Oxford Level of Evidence | Primary Tumor | CM’s Clinical Characteristics | CM’s Dermoscopic Findings |
|---|---|---|---|---|
| Chernoff et al., 2014 [1] | Level 4 | Various (breast, colorectal, thyroid, ovarian and other cancers) | Erythematous or pigmented nodules, papules, and plaques. May be ulcerated. |
Vascular pattern (serpentine, arborizing, dotted, comma-shaped vessels). Structureless or homogeneous pink appearance. Hyperpigmentation. Brown streaks, blue-gray globules. |
| Bombonato et al., 2018 [12] | Level 3 | Primary cutaneous T-cell lymphomas | MF: patches, plaques, tumors. | MF: fine short linear vessels, dotted vessels, spermatozoa-like structures, orange-yellowish patchy areas. |
| pcALCL: solitary firm nodule with rapid growth and ulceration. | pcALCL: pink-to-yellow structureless areas, polymorphous vessels. | |||
| LyP: recurrent papular, papulonecrotic or nodular lesions. | LyP: variable. | |||
| Primary cutaneous B-cell lymphomas | PCFCL: Solitary or multiple papules and plaques on the head and neck area. | PCFCL: white circles/areas, arborizing vessels, scales, salmon- colored background. |
||
| PCLBCL, LT: Solitary or multiple papules and plaques on the legs. |
PCLBCL, LT, PCMZL: polymorphous vascular pattern, arborizing vessels, scales, salmon-colored background, white circles. | |||
| PCMZL: Solitary or multiple papules and plaques on trunk and extremities. | ||||
| Cutaneous pseudolymphomas | Lymphoma cutis, Acral pseudo-lymphomatous angiokeratoma: reddish nodules, plaques | Linear vessels, reticular white lines. Follicular yellowish spots, arborizing vessels. Punctate and irregular linear vessels, white-pink structureless areas. |
||
| Pseudo-lymphomatous folliculitis: dome shaped hyperplastic hair follicles | ||||
| Kelati & Gallouj, 2018 [15] | Level 5 | Breast cancer | Pink nodules on indurated skin. Well-demarcated erythema and edematous cellulitis with central ulceration. |
Pink-orange background, yellow central areas. Linear irregular and polymorphic vessels. Whitish bright lines and structureless areas. |
| Soares et al., 2014 [16] | Level 5 | Renal carcinoma | Sharply demarcated, firm purple-red nodule. | Purple color. Linear vessels (parallel distribution in the centre combined with white lines, ramification in the periphery). |
| Bono et al., 2004 [17] | Level 3 | Melanoma | Not given. | Homogenous pattern (red, brown, gray-black uniform pigmentation). Saccular pattern (blue, red, brown, gray sacculi). Amelanotic pattern (serpentine vessels, corkscrew-like vessels, arborizing, dotted vessels; milky-red areas; angioma like lacunae, crystalline structures). Vascular pattern (punctate vessels, corkscrew vessels). Polymorphic pattern. Focal dermoscopic patterns: reddish color, peripheral spots/globules, perilesional erythema, pigmentary peripheral halo. |
| Rubegni et al., 2013 [18] | Level 3 | Melanoma | Not given. | |
| Jaimes et al., 2012 [19] | Level 4 | Melanoma (amelanotic) | Pink/erythematous papules and nodules. Ulceration/crust. |
Vascular pattern (serpentine, glomerular, hairpin, corkscrew-like, arborizing and dotted vessels, angioma-like lacunae), milky red areas. |
| Hammami-Ghorbel et al., 2014 [20] | Level 5 | Melanoma | Widespread pink to light-brown macules. | Polymorphous vascular pattern with milky red areas, saccular and dotted vessels, draft of network. |
| Wyatt et al., 2006 [21] | Level 5 | Breast cancer | Irregular pigmented, firm pink-red nodule with dark brown cerebriform centre. | Multiple small, brown globules of irregular distribution, blue-white veil, brown pigmentation. |
| De Giorgi et al., 2009 [22] | Level 5 | Thyroid cancer | Erythematous, slightly raised lesion with irregular borders. | Polymorphous vascular pattern of linear irregular and dotted vessels. |
MF: Mycosis fungoides
pcALCL: Primary Cutaneous CD30+ Anaplastic Large-Cell Lymphoma
LyP: Lymphomatoid papulosis
PCFCL: Primary Cutaneous Follicle-center Lymphoma
PCLBCL LT: Primary Cutaneous diffuse Large B-Cell Lymphoma, Leg Type
PCMZL: Primary Cutaneous Marginal-Zone B-Cell Lymphoma.
4. DISCUSSION
Although skin metastases are quite rare and often portend a poor prognosis, they are still of great clinical importance. Cutaneous metastases may arise from various primary tumors (melanoma [17-20], breast cancer [1, 15, 21], thyroid cancer [1, 22], lymphomas [12], and others [2-6, 16]). Their clinical appearance varies from erythematous to pigmented papules or nodules with or without ulceration [1, 3-6, 12-22].
Overall, the use of dermoscopy increases yearly, but case reports and studies on the specific dermoscopic features of cutaneous metastases are not that common. This could be due to the uncommonness of secondary cutaneous malignancies in general or the lack of dermoscopy skills and use in other specialties, excluding the dermatology field. The most structured information in the literature on the dermoscopic findings in specific tumor metastases is related to melanoma [17, 18] and cutaneous lymphomas [12]. This could be due to the fact that these patients are often overseen by dermatologists, who tend to use dermoscopy as a diagnostic tool more than any other specialists.
The main reported characteristics of skin metastases in dermoscopy overall are various forms of vascular patterns (ex. linear, dotted, arborizing, corkscrew-like and others, as well as a mix of various patterns or polymorphous vessels) [1, 12-22]. The vascular patterns may be related to the thickness of the secondary metastases [18]. Other dermoscopic signs include structureless areas, white lines, and the presence of peripheral globules or spots. Changes in color (hyperpigmentation, pink, yellow and orange patches) are also described [1, 12-22].
As breast cancer is one of the most common types of cancer to metastasize the skin in the female population [3], we think that more in-depth information should be gathered regarding the various clinical forms and their dermoscopic patterns, as the data varies in case reports. Clinical variants from the pink nodular lesion with erythema and polymorphic vessels with bright white lines seen in dermoscopy [15] and structures with pigmentation and globules of irregular distribution with a blue-white veil [1, 21], mimicking melanoma appearance, are reported. These patterns should be analysed more thoroughly and characterized accordingly.
The results of this review are of importance to dermatologists and oncologists and other specialists, whose patients have a clinical suspicion of cutaneous metastases, to verify or deny their concerns before receiving lesion biopsy results. Limitations of these results include the factor that most of the results were obtained from case reports and case series studies (Oxford Level of Evidence 5 and 4). Multicentric studies on large populations with cutaneous metastases would be useful to better distinguish the dermoscopic characteristics of cutaneous metastasis.
CONCLUSION
Cutaneous metastases are rare in everyday practice but are crucial to recognize. Their main clinical characteristics are pink to erythematous nodules with vascular patterns seen on dermoscopy, though other variants with different types of primary lesions and color patterns are reported. As the use of dermoscopy rises yearly, a better understanding of the dermoscopic features in cutaneous metastases should be obtained and reported, performing larger studies with standardized description criteria.
CONSENT FOR PUBLICTION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


