All published articles of this journal are available on ScienceDirect.
Efficacy of Autologous Serum Therapy in Chronic Urticaria, A Prospective Experimental Cohort Study
Abstract
Background:
Chronic Urticaria is one of the most therapeutic difficulties confronted by a dermatologist.
Objective:
The objective of this study is to estimate the efficacy of Autologous Serum Therapy (AST) in Chronic Urticaria patients and compare the efficacy of AST in each group of Autologous Serum Skin Test (ASST) positive and negative.
Methods:
A prospective study was conducted for the period of one year (July 2020 – July 2021) at Tishreen University Hospital. We enrolled 50 patients suffering from Chronic Urticaria. ASST was done for all patients before the treatment. We gave patients 9 injections of Autologous Serum intramuscularly once a week. The response to AST was evaluated by using Urticaria Total Severity Score (UTSS) before the treatment (0 weeks), after the treatment (9 weeks), and followed up for 3 months after the end of the treatment (21 weeks).
Results:
Between 50 patients, the sample was distributed equally in each group of ASST (positive and negative). 18 patients (36%) had an excellent response, 7 patients (14%) had a very good response, 11 patients (22%) had a good response, and 14 patients (28%) had no response. The treatment was effective in both ASST positive and negative groups, however, there was no significant relationship in response between the two groups.
There was a significant relationship between clinical response and severity of disease p-value = 0.04, the majority of excellent response cases (88.9%) had severe disease before the treatment.
Limitations:
The main limitations of our study were the lack of patients and the short follow-up period (12 weeks).
Conclusion:
AST was effective in treating Chronic Urticaria without side effects. There was a significant response in both ASST positive and negative groups.
1. INTRODUCTION
Chronic Urticaria (CU) is a popular and disturbing dermatosis defined by the occurrence of wheal on most days of the week, constantly for 6 weeks or more [1]. In the United States and five European countries, the point prevalence of CU diagnosis is 0.53% and 0.63%, respectively. CU may arise at any age; however, it is most frequent between (20-40) years [2]. CU is divided into Idiopathic or Inducible. Chronic Idiopathic Urticaria represents up to 80% to 90% of CU patients. Chronic Inducible Urticaria is less popular and requires specific triggers which may be medications, physical stimuli to occur [3].
Approximately 30 – 40% of patients with CU have autoantibodies directed towards IgE (itself and/or its high-affinity receptor FcεRI) and are categorized having Chronic Autoimmune Urticaria [4].
The activation and degranulation of mast cells lead to the release of many inflammatory mediators, especially histamine. This results in vasodilatation and an increase in vascular permeability [5]. Lately, IL-24 may play a major role as a functional autoantigen of IgE autoantibodies in CSU [6]. The simplest screening method to define patients with CAU is ASST [7].
Since 1913, Autologous Whole Blood injection (AWB) was used to treat a variety of dermatologic conditions, including urticaria [8]. We replaced the Whole Blood with the Autologous Serum for the following reasons:
1. The circulating autoreactive factors aren't present in cellular components, however, in the serum.
2. A finer needle can be used for injecting the serum which makes the treatment less painful for the patients.
3. The Whole Blood must be injected as fast as possible after being drawn to avoid clotting [9].
Our study aimed to estimate the efficacy of Autologous Serum Therapy (AST) in Chronic Urticaria patients and compare the efficacy of AST in each group of Autologous Serum Skin Test (ASST) positive and negative.
2. MATERIALS AND METHODS
This is a prospective observational cohort study of a group of patients with Chronic Urticaria attending the Dermatology Department's outpatient clinic at Tishreen University Hospital in Lattakia-Syria for one year (July 2020 to July 2021). 50 patients with Chronic Urticaria who received treatment with Autologous Serum Therapy (AST) were included in the study. Institutional review board (IRB) approval was secured before the study and written informed consent was also obtained from all the patients in advance. The study included patients above 18 years who had Chronic Urticaria for 6 weeks or more. All patients were previously treated with antihistamines without response. None of the patients were previously treated with omalizumab because the drug was unavailable in our hospital. We excluded pregnant, lactating women and those immunosuppressed patients due to medication or disease. We did Autologous Serum Skin Test (ASST) for all patients before the treatment. We gave the patients 9 injections of Autologous Serum intramuscularly once a week. The response to AST was evaluated by using Urticaria Total Severity Score (UTSS) before the treatment (0 weeks), after the treatment (9 weeks), and followed up for 3 months after the end of the treatment (21 weeks). Based on these, a 0-18 total severity score was created. We categorized the severity of disease as clear (TSS=0), mild (TSS 1-6), moderate (TSS 7-12), or severe (TSS 13-18).
Tables 1 and 2 show the Urticaria Total Severity Score (UTSS) and the response to AST, respectively [1, 10]. During this treatment short-acting antihistaminic drugs (pheniramine) were allowed only in necessary cases [1].
2.1. Autologous Serum Skin Test
5 ml of blood was collected and centrifuged at 3000 r.p.m for 10 min to obtain serum [11]. Then we injected intradermally 0.1 ml of Autologous Serum and 5 cm away 0.1 ml of Normal Saline. We read the results after 30 minutes. When the wheal diameter at the Autologous Serum site was 1.5 mm larger than the Normal Saline site, we considered the ASST positive [1].
| Parameter | Score | |||
|---|---|---|---|---|
| - | 0 | 1 | 2 | 3 |
| Number of wheals | None | ≤ 10 | 11 – 50 | >50 |
| Size of wheals | None | <1 cm | 1 – 3 cm | >3 cm |
| Intensity of pruritus | None | Mild | Moderate | Severe |
| Duration of persistence | None | 1 h | 1 – 12 h | >12 h |
| Frequency of appearance | None | <once or once a week | 2 – 3 times a week | Daily, almost daily |
| Frequency of antihistamine use | None | <once or once a week | 2 – 3 times a week | Daily, almost daily |
| Response to AST | UTSS Reduction before & after AST |
|---|---|
| Excellent response | UTSS = 0 |
| Very good response | UTSS reduction from severe to mild grade |
| Good response | UTSS reduction from severe to moderate or moderate to mild grade |
| Nil | No change in UTSS |
To avoid false-negative results:
(1) The patients should be off short-acting antihistamines, long-acting antihistamines, and doxepin for at least 2-3 days, 7 days, and 2 weeks, respectively, before the test.
(2) The study also excluded patients taking immunosuppressive agents (for example, corticosteroids) within 6-12 weeks before the test.
(3) When ASST was done, the patient should have active urticaria. However, we have to avoid the regions where wheals have appeared in the last 24 hours [12].
2.2. Autologous Serum Therapy
2 ml of the serum was given to the gluteus muscle. We gave the patients 9 injections of Autologous Serum intramuscularly once a week [1]. We don't define the precise mechanism of how AST works may be the premise of Autologous Serum that contains tolerance-producing anti-idiotype antibodies to the mast cell degranulating antigens [5].
2.3. Statistical Analysis
Statistical analysis was performed by using the IBM SPSS version20. Basic Descriptive statistics included means, standard deviations (SD), median, frequency, and percentages. Differences of distribution were examined by using Fisher's exact test. Wilcoxon test was used to compare two paired groups, and one-way analysis of variance (ANOVA) to compare responses across groups. P-value ≤0.05 was considered statistically significant.
3. RESULTS
50 patients with CU who presented to the Department of Dermatology from July 2020 to July 2021 and met our criteria of inclusion, participated in the study. (Table 3) shows the baseline characteristics.
The median age of patients who enrolled in the study was 31.5 years, 64% of the patients were females. Most cases of CU patients were severe (78%), and the median duration of the disease was 1.5 years. Between 50 patients, the sample was distributed equally in each group of ASST (positive and negative). 18 patients (36%) had an excellent response, 7 patients (14%) had a very good response, 11 patients (22%) had a good response, and 14 patients (28%) had no response.
78%, 18%, and 4% had severe, moderate, and mild disease, respectively.
At the end of treatment,13 patients (26%) had an excellent response and 22 patients (44%) had a good response. At follow-up 3 months after the end of treatment, 18 patients (36%) had an excellent response and 11 patients (22%) had a good response. There was a significant reduction in UTSS before and after the treatment (p-value<0.05) (Fig. 1).
Among 25 ASST positive patients, 13 patients (52%) had an excellent response, 2 patients (8%) had a very good response, 5 patients (20%) had a good response, and 5 patients (20%) had no response. Among 25 ASST negative patients, 5 patients (20%) had an excellent response, 5 patients (20%) had a very good response, 6 patients (24%) had a good response, and 9 patients (36%) had no response. There was significant response to AST in both ASST positive and negative groups (p-value<0.05). 80%, 64% had a response to AST in ASST positive and negative groups, respectively. Therefore, there was no relationship between the two groups (p-value>0.05).
Over the next 12 weeks, UTSS continued to show a reduction in ASST (+) and ASST (–) groups. The patients with excellent responses were distributed in (52%) and (20%) in ASST (+) and (-) groups, respectively, with statistically significant (p-value=0.02) (Fig. 2).
| Results | Variables |
|---|---|
| (%36) 18 (%64) 32 |
Sex Male Female |
| 31.5 years (67 – 18) | Age (years) |
| (%54) 27 (%30) 15 (%16) 8 |
Duration of disease(years) 2 > years 2 – 5 years 5 < years |
| (%78) 39 (% 18) 9 (%4) 2 |
Severity of Disease Severe moderate mild |
| (%50) 25 (%50) 25 |
ASST Test Positive Negative |
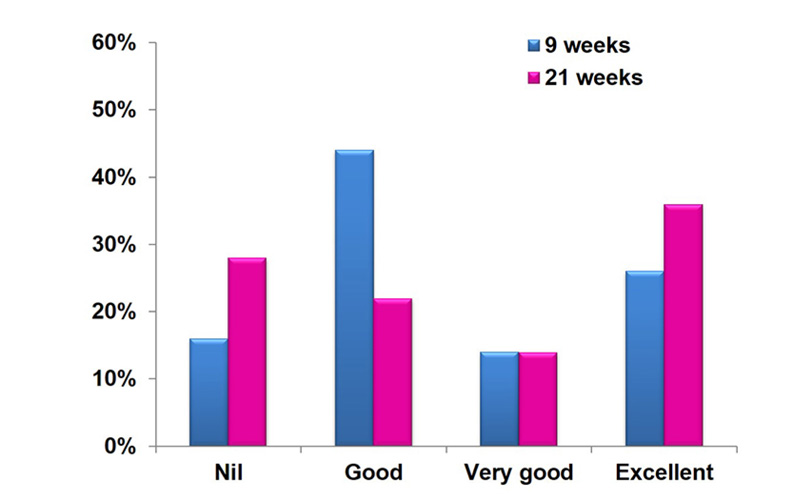
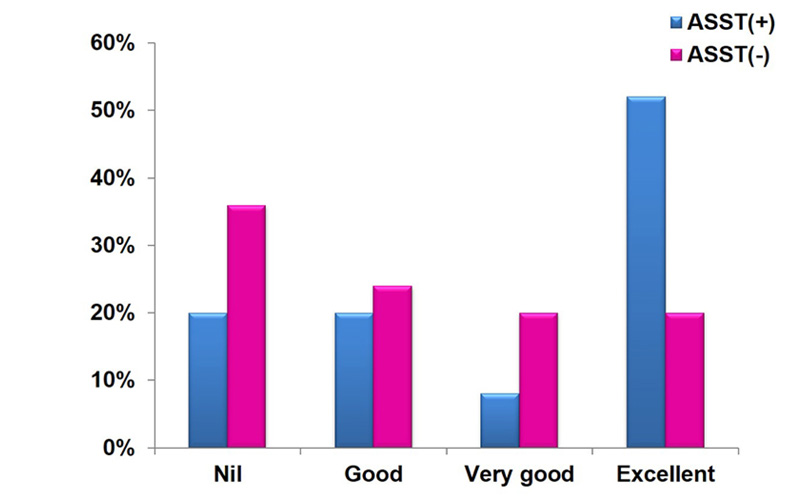
As shown in Table 4, there was no significant relationship between clinical response and age (p-value > 0.05). Also, no significant relationship between clinical response and duration of disease (p-value > 0.05) was noticed. However, there was a significant relationship between clinical response and severity of disease (p-value = 0.04). The majority of excellent response cases (88.9%) had a severe disease before the treatment.
| p-value | Excellent | Very good | Good | Nil | Variables |
|---|---|---|---|---|---|
| 0.7 | 5 (%27.8) 4 (%22.2) 6 (%33.3) 2 (%11.1) 1 (%5.6) |
4 (%57.1) 2 (%28.6) 1 (%14.3) 0 (%0) 0 (%0) |
3 (%27.2) 4 (%36.4) 2 (%18.2) 1 (%9.1) 1 (%9.1) |
7 (%50) 4 (%28.6) 1 (%7.1) 2 (%14.3) 0 (% 0) |
Age 18 – 27 28 – 37 38 – 47 48 – 57 58 – 67 |
| 0.5 | 10 (% 55.6) 4 (%22.2) 4 (%22.2) |
4 (%57.1) 3 (%42.9) 0 (%0) |
4 (%36.4) 4 (%36.4) 3 (%27.2) |
9 (%64.3) 4 (%28.6) 1 (%7.1) |
Duration of disease years<2 2-5 years years>5 |
| 0.04 | 0 (%0) 2 (%11.1) 16 (%88.9) |
0 (%0) 0 (%0) 7 (%100) |
0 (%0) 2 (%18.2) 9 (%81.8) |
2 (%14.3) 5 (%35.7) 7 (%50) |
Severity of disease Mild Moderate Severe |
4. DISCUSSION
Chronic Urticaria is a nuisance dermatosis of skin that is very itchy. The treatment with AST has proven to be an inexpensive and effective way to reduce medication intake and improve patients' quality of life. Because there is no standardized treatment for CU, there is ongoing research for the newer method to offer long-term remission with fewer fees and side effects. We observed that AST was an effective method in both ASST groups (positive and negative). None of the patients reported any side effects.
In a study by Parekh et al. in 2017, 39 patients were given 9 injections of AST intramuscularly once a week and the follow-up period was 6 weeks after the end of treatment (15 weeks). 5 patients (13.8%) had an excellent response. 11 patients (28.2%) had an observed response. In our study, 18 patients (36%) had excellent response, and 7 patients (14%) had a very good response. This may be due to the short follow-up period in Parekh et al. study. Similar to our study, there was no significant relationship in response between the two groups of ASST (positive and negative). Also, no side effects were reported [13].
In a study by Aruna et al. in 2017, 61 patients were given 8 injections of AST intramuscularly once a week and the follow-up period was 3 months after the end of treatment (21 weeks). 10 patients (16.4%) had an excellent response, and 20 patients (32.8%) had a good response. In our study, 18 patients (36%) had an excellent response, and 7 patients (14%) had a very good response. This may be due to lower doses of AST in Aruna et al. study [14].
In a study done by Surendran et al. in 2019, 30 patients were given 9 injections of AST intramuscularly once a week and the follow-up period was (3 – 4 months) after the end of treatment (21 - 25 weeks). 8 patients had a very good response, 13 patients had a good response, however in our study 18 patients (36%) had an excellent response, 7 patients (14%) had a very good response. The lack of patients in Surendran et al. study may be the possible reason behind the difference from our study. Similar to our study, there was a significant response to AST in both ASST positive and negative groups [10].
In a study by Kumaravel et al. in 2017, 41 patients were given 9 injections of AST intramuscularly once a week and the follow-up period was 3 months after the end of treatment (21 weeks). 7 patients had an excellent response versus 18 patients (36%) in our study. This may be due to the increased number of severe cases in our study [15].
LIMITATIONS
The main limitations of our study were the low number of patients and short follow-up period (12 weeks).
CONCLUSION
AST is a safe, worth trying and effective method, especially in severe cases. Further studies with larger sample sizes and longer follow-up periods are recommended.
LIST OF ABBREVIATIONS
| AST | = Autologous Serum Therapy |
| ASST | = Autologous Serum Skin Test |
| UTSS | = Urticaria Total Severity Score |
| CU | = Chronic Urticaria |
| CAU | = Chronic Autoimmune Urticaria |
| AWB | = Autologous Whole Blood |
| SD | = Standard Deviations |
| ANOVA | = Analysis of Variance |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Institutional review board (IRB) approval was secured before the study.
HUMAN AND ANIMAL RIGHTS
No animals were used for studies that are the basis of this research. All the humans were used in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013 (http://ethics.iit.edu/ecodes/node/3931).
CONSENT FOR PUBLICATION
A written informed consent was also obtained from all the patients in advance.
STANDARDS FOR REPORTING
STROBE guidelines were followed.
AVAILABILITY OF DATA AND MATERIALS
The data sets used and/or analysed during this study are available from the corresponding author upon request.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


