REVIEW ARTICLE
Cutibacterium Acnes as a Cause of Post-surgical Prosthesis Infection
Emma Scott1, *, Craig Burkhart2, 3
Article Information
Identifiers and Pagination:
Year: 2023Volume: 17
E-location ID: e187437222309130
Publisher ID: e187437222309130
DOI: 10.2174/0118743722258402230920113212
Article History:
Received Date: 05/05/2023Revision Received Date: 23/07/2023
Acceptance Date: 22/08/2023
Electronic publication date: 20/10/2023
Collection year: 2023

open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Cutibacterium acnes (C. acnes) is most well known for its role in the skin disease acne; however, it is becoming increasingly recognized as a cause of post-surgical prosthesis infection [1]. This paper aims to discuss the characteristics of C. acnes and how it relates to post-surgical prosthesis infections.
1. BACKGROUND OF C. ACNES
Historically, C. acnes has been thought of as a commensal bacterium but has started to be recognized as an opportunistic pathogen [1]. C. acnes originally was included in the Bacillus genus, then was included in the Corynebacterium genus. Then, due to the bacterium's ability to produce propionic acid as a product of anaerobic metabolism, it was assigned to the Propionibacterium genus. The Propionibacterium genus has been renamed the Cutibacterium genus, and the bacterium is currently referred to as Cutibacterium acnes [1] (Fig. 1).
C. acnes is found primarily in the sebaceous glands of the skin [1] but also in other areas rich in sebaceous glands, such as the conjunctiva, intestinal tract, oral mucosa, and the external auditory canal [2]. It is involved in illnesses, including endocarditis, endophthalmitis, prostatitis, sarcoidosis, synovitis, acne vulgaris, osteitis syndrome, osteomyelitis, and septic arthritis [3].
C. acnes is a lipophilic, gram-positive, rod-shaped [1], non-spore-forming, facultative anaerobic bacteria [4]. While C. acnes is primarily anaerobic, it does possess all of the necessary proteins for oxidative phosphorylation, which allows it to survive in oxygen-rich environments [1]. Additionally, C. acnes has a cell wall and envelope containing various lipids, and the cell wall contains peptidoglycan. C. acnes also contains lipoglycans that have a lipid anchor of fatty acids and contain substantial concentrations of mannose, glucose, and galactose [1]. The makeup of the C. acnes cell wall increases the bacterium's resistance to oxidizing enzymes, which allows for intracellular survival [5].
2. C. ACNES POST-SURGICAL INFECTION BACKGROUND
C. acnes is considered commensal on healthy skin but is implicated in foreign body infections [6], such as cerebrospinal fluid shunt [7] or prosthetic device. It can invade the deep tissues via a surgical incision, but it is suspected that the manipulation of soft tissue by the surgeon or instruments helps the bacterium spread [8].
C. acnes is found in over 50% of post-surgical prosthesis infections [1] and is the most common cause of shoulder prosthetic joint infections [4], accounting for roughly 56% of surgical shoulder infections [5]. Additionally, C. acnes is present in the superficial and deep tissues of 20% of all primary shoulder arthroplasties [9].
Post-surgical C. acnes infections occur most frequently at surgical sites rich in sebaceous glands [4]. These infections can occur at the time of surgery [10] but typically occur within 3 to greater than 24 months after implant placement [2]. The infection can be diagnosed by removing the implant, performing sonication, and allowing the culture to cultivate for up to 14 days [4].
Post-surgical C. acnes infections can occur because surgical antiseptic prep only lasts 30-180 minutes, after which the bacteria can begin to regrow along the incision [4]. After C. acnes enters the incision site, the bacterium must move toward and adhere to the implant [4].
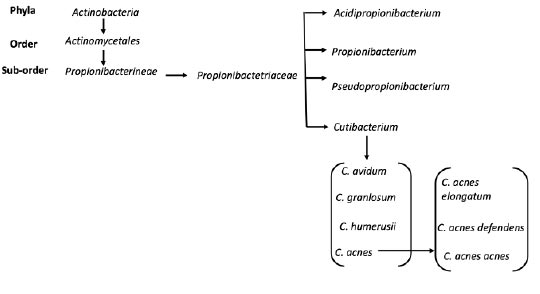 |
Fig. (1). Classification of C. acnes diagram [1]. |
3. VIRULENCE FACTORS OF C. ACNES
C. acnes has many virulence factors that aid in its pathogenicity. One such virulence factor is the production of a biofilm [1]. A biofilm is a bacterial extracellular matrix made up of polysaccharides, proteins, and extracellular DNA [1]. C. acnes biofilms form in the pilosebaceous unit and can aid in the adherence of keratinocytes to one another. This allows stronger adhesion of the bacterium to the walls of the follicle [1]. C. acnes biofilms can also form on many different biomaterials, making implants susceptible to C. acnes colonization [4]. When an implant is placed, the host will cover the device with extracellular matrix proteins, and the bacteria will adhere to these proteins. The granulocytes surrounding the implant may have decreased activity, thus inhibiting their ability to eliminate the bacterium [4].
Another virulence factor of C. acnes is the possession of lipases [1]. Lipases metabolize sebum, releasing free fatty acids into the pilosebaceous unit, which can induce inflammation. Because lipases break down lipids, the lipid levels determine lipase activity. This makes C. acnes more pathogenic in highly lipophilic environments [1]. Lipases also allow C. acnes bacteria to adhere to both each other and other surfaces, which can further aid in its adherence to medical implants [1]. Two specific lipases present in C. acnes are glycerol-ester-hydrolase A (GehA) and glycerol-ester-hydrolase B (GehB), which are both densely concentrated in sebaceous follicles [1].
Hyaluronate lyase also aids in C. acnes' pathogenicity. Hyaluronate lyase breaks down hyaluronic acid in the epidermis and dermis extracellular matrix [1]. The products of hyaluronic acid break down by hyaluronate lyase enzymes provide nutrients for the bacterium and further contribute to inflammation. Also, by breaking down the upper layers of the skin and extracellular matrix, hyaluronate lyase allows inflammation to spread [1]. Furthermore, different strains of C. acnes express different hyaluronate lyase variants [1]. For example, C. acnes produces both the HYL-IB/II variant and the HYL-IA variant. HYL-IB/II is highly active and completely breaks down the hyaluronic acid in the type IB and II strains [1]. HYL-IA is less active and can only partially break down the hyaluronic acid in type IA strains [1]. The different hyaluronate lyase expression in the different C. acnes strains, allowing the different strains to invade different tissues [1]. This provides reasoning for why type AI is associated with inflammatory acne of the skin, while type IB/II is associated with deep soft tissue infections [11].
DsA1 protein is another virulence factor of C. acnes. DsA1 is both an adhesion protein and a fibrinogen-binding protein. By promoting the clumping of fibrinogen, DsA1 can aid in the bacterium's adaptability and allow it to survive in the pilosebaceous unit environment [1].
Christie-Atkins-Munch-Peterson (CAMP) factors are toxin proteins that can cause host tissue damage by creating pores in host membranes [1] via binding to IgG and IgM [12]. CAMP factors can also kill sebocytes in the sebaceous gland, triggering inflammation by inducing cytolysis and cytokine secretion [12].
Additionally, the radical oxygenase (RoxP) reduces free radicals and helps the bacterium survive in the oxygen-rich environment of the skin [1]. Lastly, sialidase and pili/fimbriae aid in adhesion and colonization [1].
4. RISK FACTORS FOR DEVELOPING POST-SURGICAL C. ACNES INFECTIONS
There are various risk factors for C. acnes post-surgical prosthesis infections. First of all, C. acnes infections are more common in shoulder arthroplasties than in hip or knee arthroplasties because of the higher concentration of sebaceous glands [4]. People under the age of 40 years also have a higher burden of C. acnes. This can be due to sebaceous glands decreasing with age, which adds an element of protection for older individuals [5]. These infections are also more common in men than women because men have more sebaceous glands [13]. Additionally, C. acnes colonizes hair follicles, so increased hairiness is associated with an increased C. acnes burden [5]. Neither diabetes nor smoking have been proven to be associated with a higher C. acnes burden; however, they can be associated with an increased risk of poor outcomes with a C. acnes infection due to the weakened immune response [5].
4.1. Diagnosis
The diagnosis of C. acnes post-surgical prosthesis infections can prove difficult. For example, it does not display the common signs of inflammation, such as edema and drainage [13]. There is also a lack of systemic symptoms. In fact, in the absence of systemic symptoms, joint pain is the most common presenting complaint from patients [14].
Additionally, it is likely underdiagnosed due to the short incubation time of standard lab cultures [15]. In a minor C. acnes infection, inflammatory biomarkers are typically normal, and histopathology can show no acute inflammation, indicative of low virulence and bacterial burden.
C. acnes takes roughly 14 days to grow, so doing only a joint aspiration can result in treatment delays [15]. It is also not evenly distributed, further making it difficult to culture. Therefore, 3 to 6 tissue samples should be obtained for diagnosis using aerobic and anaerobic cultures at the time of explantation surgery. The multiple samples should account for the varying distribution of the biofilm on the device and rule out the possibility of contamination from the skin [4]. Also, if possible, antibiotics should not be used for two weeks before sample collection to increase sensitivity [4]. When the implant is removed, another recommended technique for diagnosis is sonication. Sonication is effective at detaching the bacteria from the surface of the implanted device and has proven to be more sensitive than vortexing alone [4]. Sonication is also more sensitive than standard tissue culture [15]. Alternatively, C. acnes infections can be detected preoperatively. For example, synovial IL-6, calprotectin, or a combination of IL-6, IL-12 and TNF-a show greater than 75% sensitivity and 85% specificity for detecting C. acnes infections preoperatively [16].
4.2. Treatment
Post-surgical C. acnes prosthesis infections should be treated similarly to other implant infections [4]. First, one should surgically remove the implant and thoroughly debride the area of all infected tissue. Then, susceptibility testing should be performed to determine the best antibiotic to use due to increasing antibiotic resistance [4]. 3 to 6 months of antibiotic treatment should be administered, including 2 to 6 weeks of intravenously administered beta-lactam [4]. Rifampin is also typically included in the antibiotic regimen because it is active against the biofilm, and if the particular C. acnes is susceptible to rifampin, the time between device removal and reimplantation can be decreased [15]. Clindamycin is another antibiotic widely used in the treatment of C. acnes due to its high bioavailability and sufficient bone diffusion [16, 17]. A study investigating the treatment of post surgical C. acnes prosthesis infections found that surgical revision and prolonged antibiotics are effective treatments for 97% of patients [18].
Photodynamic therapy is also being investigated as a possible treatment for these infections. Photodynamic therapy uses photosensitizers, molecules sensitive to light and ultraviolet or visible light. When the light of a specific wavelength activates the photosensitizer, it reacts with oxygen. This creates a reactive oxygen species, which can cause cell death [19]. There is evidence suggesting blue light's efficacy in treating acne vulgaris, which is also caused by C. acnes. In light of this, there is emerging evidence for this treatment method for post-surgical prosthesis infections. For example, one study found that blue light plus demeclocycline is an effective treatment for C. acnes post-surgical prosthesis infections [19]. However, after the use of photodynamic therapy, C. acnes frequently recolonizes within 7-21 days. Therefore, it is recommended that it be applied on the day of surgery, but this therapy can also induce skin inflammation [3]. Because of the erythema, same-day photodynamic therapy can also pose issues for surgery [3].
4.3. Antibiotic Resistance
As with many bacterial pathogens, C. acnes is gaining increasing antimicrobial resistance. Due to rRNA point mutations, strains of C. acnes have become resistant to erythromycin, clindamycin, and tetracycline [4]. Macrolides and tetracyclines have also been largely used in the treatment of acne vulgaris, which contributes to increasing resistance [20]. Additionally, anaerobic bacteria can have an innate resistance to certain antibiotics because they do not possess the mechanisms needed to take up the antibiotic. Specifically, C. acnes has an innate resistance to Fosfomycin [15] and metronidazole [21].
Biofilms also play an important role in antibiotic resistance. Up to 80% of human bacterial and fungal infections involve a microbial biofilm. The biofilm allows the microbe to persist in varying and harsh environments, but it also increases microbial resistance. In fact, microbes protected by a biofilm can resist 10-1000 times more antibiotics than microbes without biofilms [22]. Also, because there is a high cellular density in biofilms, horizontal gene transfer is common. This leads to a heightened mutation rate. For example, rifampin is widely used in C. acnes post-surgical infections because it targets the bacterium's biofilm. However, C. acnes is also gaining resistance to rifampin via a point mutation in the rpoB gene [2]. The increasing antibiotic resistance of C. acnes poses a strong argument for susceptibility testing before starting an antibiotic regimen to most effectively and efficiently treat these potentially serious infections [2].
4.4. Economic Impact
In the United States, there are an estimated 500,00 surgical site infections yearly [23], accounting for 17% of nosocomial infections [24]. Surgical site infections can lead to a $1-10 billion increase in direct and indirect healthcare costs in one year, with a greater cost being attributed to infections occurring after discharge [23]. Because C. acnes has a relatively long incubation period, it typically occurs after discharge, making these costs especially relevant. Compared to infections diagnosed before discharge, infections occurring after discharge have an increased cost of outpatient and inpatient care as well as increased pharmacy and radiology costs. These patients also require more home health care and medical equipment, further increasing costs [23]. It is estimated that the cost of treating post-surgical prosthesis infections is five times greater than that of uncomplicated arthroplasties [24]. In fact, A prosthetic joint infection incurs an average hospital charge of $106,311, and between the years 2011 and 2018, the costs due to these infections increased by more than 300% [25]. Furthermore, these charges are expected to increase an additional 176% by 2030 [25].
CONCLUSION
In conclusion, C. acnes has been recognized as a common cause of post-surgical prosthesis infections due to various virulence factors, including biofilm production, lipases, hyaluronate lyases, DsA1 protein, CAMP factors, RoxP, sialidase, and pili/fimbriae [1]. Host risk factors, such as age, sex, site of joint replacement, hairiness, smoking and diabetes, also affect post-surgical C. acnes infections [5]. With the risk this pathogen poses, it is important for rapid diagnosis and treatment, which is made difficult by being slow to culture. Lastly, C. acnes is gaining antibiotic resistance, so it is important to perform antibiotic susceptibility testing before initiating treatment. Additionally, prevention and swift resolution of these infections will lighten their economic impact.
LIST OF ABBREVIATIONS
| CAMP | = Christie-Atkins-Munch-Peterson |
| GehA | = glycerol-ester-hydrolase A |
| GehB | = glycerol-ester-hydrolase B |
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
Dr. Craig Burkhart is the EIC of The Open Dermatology Journal.
ACKNOWLEDGEMENTS
Declared none.
REFERENCES
| [1] | Mayslich C, Grange PA, Dupin N. Cutibacterium acnes as an opportunistic pathogen: An update of its virulence-associated factors. Microorganisms 2021; 9(2): 303. |
| [2] | Aubin GG, Portillo ME, Trampuz A, Corvec S. Propionibacterium acnes, an emerging pathogen: From acne to implant-infections, from phylotype to resistance. Med Mal Infect 2014; 44(6): 241-50. |
| [3] | Waldmann I, Schmid T, Prinz J, et al. Photodynamic therapy improves skin antisepsis as a prevention strategy in arthroplasty procedures: A pilot study. Photodiagn Photodyn Ther 2020; 31: 101941. |
| [4] | Achermann Y, Goldstein EJC, Coenye T, Shirtliff ME. Propionibacterium acnes: From commensal to opportunistic biofilm-associated implant pathogen. Clin Microbiol Rev 2014; 27(3): 419-40. |
| [5] | Kaveeshwar S, Duvall G, Jones DL, et al. Risk factors for increased shoulder Cutibacterium acnes burden. JSES Int 2020; 4(3): 464-9. |
| [6] | Aubin GG, Baud’huin M, Lavigne JP, et al. Interaction of Cutibacterium ( formerly Propionibacterium) acnes with bone cells: A step toward understanding bone and joint infection development. Sci Rep 2017; 7(1): 42918. |
| [7] | Mongaret C, Velard F, Reffuveille F. Cutibacterium acnes : The urgent need to identify diagnosis markers. Infect Immun 2021; 89(4): e00753-20. |
| [8] | Falconer TM, Baba M, Kruse LM, et al. Contamination of the surgical field with Propionibacterium acnes in primary shoulder arthroplasty. J Bone Joint Surg Am 2016; 98(20): 1722-8. |
| [9] | Torrens C, Bellosillo B, Gibert J, et al. Are Cutibacterium acnes present at the end of primary shoulder prosthetic surgeries responsible for infection? Prospective study. Eur J Clin Microbiol Infect Dis 2022; 41(1): 169-73. |
| [10] | Nodzo SR, Boyle KK, Bhimani S, Duquin TR, Miller AO, Westrich GH. Propionibacterium acnes host inflammatory response during periprosthetic infection is joint specific. HSS J 2017; 13(2): 159-64. |
| [11] | Kraaijvanger R, Veltkamp M. The role of cutibacterium acnes in sarcoidosis: From antigen to treatable trait? Microorganisms 2022; 10(8): 1649. |
| [12] | Wang Y, Hata TR, Tong YL, et al. The anti-inflammatory activities of Propionibacterium acnes camp factor-targeted acne vaccines. J Invest Dermatol 2018; 138(11): 2355-64. |
| [13] | Elston MJ, Dupaix JP, Opanova MI, Atkinson RE. Cutibacterium acnes (formerly Proprionibacterium acnes) and Shoulder Surgery. Hawaii J Health Soc Welf 2019; 78(11)(2): 3-5. |
| [14] | Vilchez H, Escudero-Sanchez R, Fernandez-Sampedro M, et al. Prosthetic shoulder joint infection by cutibacterium acnes: Does rifampin improve prognosis? A retrospective, multicenter, observational study. Antibiotics 2021; 10(5): 475. |
| [15] | Portillo ME, Corvec S, Borens O, Trampuz A. Propionibacterium acnes: An underestimated pathogen in implant-associated infections. BioMed Res Int 2013; 2013: 1-10. |
| [16] | Pruijn N, Schuncken ACH, Kosse NM, Hofstad CJ, Dorrestijn O. Pre- and peroperative diagnosis of Cutibacterium acnes infections in shoulder surgery: A systematic review. Shoulder Elbow 2021; 13(2): 131-48. |
| [17] | Courdurié A, Lotte R, Ruimy R, et al. Clindamycin efficacy for cutibacterium acnes shoulder device-related infections. Antibiotics 2022; 11(5): 608. |
| [18] | Hoch A, Fritz Y, Dimitriou D, et al. Treatment outcomes of patients with Cutibacterium acnes-positive cultures during total joint replacement revision surgery: A minimum 2-year follow-up. Arch Orthop Trauma Surg 2022; 143(6): 2951-8. |
| [19] | Bhargava S, Boyle K, Diletti S, Nodzo S, Crane J, Duquin T. 386. blue light reduces cutibacterium (Propionibacterium) acnes bacterial burden: Orthopedic shoulder infection prevention strategy? Open Forum Infect Dis 2019; 6(2): S199-200. |
| [20] | Zhang J, Yu F, Fu K, et al. C. acnes qPCR-based antibiotics resistance assay (acquire) reveals widespread macrolide resistance in acne patients and can eliminate macrolide misuse in acne treatment. Front Public Health 2022; 10: 787299. |
| [21] | Boisrenoult P. Cutibacterium acnes prosthetic joint infection: Diagnosis and treatment. Orthop Traumatol Surg Res 2018; 104(1): S19-24. |
| [22] | Hu X, Huang YY, Wang Y, Wang X, Hamblin MR. Antimicrobial photodynamic therapy to control clinically relevant biofilm infections. Front Microbiol 2018; 9: 1299. |
| [23] | Perencevich EN, Sands KE, Cosgrove SE, Guadagnoli E, Meara E, Platt R. Health and economic impact of surgical site infections diagnosed after hospital discharge. Emerg Infect Dis 2003; 9(2): 196-203. Available from: https://wwwnc.cdc.gov/eid/article/9/2/02-0232_article (Retrieved May 4, 2023). |
| [24] | Akindolire J, Morcos MW, Marsh JD, Howard JL, Lanting BA, Vasarhelyi EM. The economic impact of periprosthetic infection in total hip arthroplasty. Can J Surg 2020; 63(1): E52-6. |
| [25] | Schick S, Elphingstone J, Murali S, et al. The incidence of shoulder arthroplasty infection presents a substantial economic burden in the United States: A predictive model. JSES Int 2023; 7(4): 636-41. |