All published articles of this journal are available on ScienceDirect.
Detection of TGF-β1, HGF, IGF-1 and IGF-1R in Cleft Affected Mucosa of the Lip
Abstract
Background:
Orofacial clefts are one of the most common birth defects with multifactorial and only partly understood morphopathogenesis.
Objective:
The aim of this study was to evaluate the presence of TGF-β1, HGF, IGF-1 and IGF-1R in cleft affected mucosa of the lip.
Methods:
Lip mucosa tissue samples were obtained during surgical cleft correction from seven 2 to 6 months old children. Prepared tissue sections were stained by immunohistochemistry for TGF-β1, HGF, IGF-1 and IGF-1R. The intensity of staining was graded semiquantitatively.
Results:
We found numerous TGF-β1 and HGF-containing epithelial and connective tissue cells, moderate number of IGF-1 immunoreactive cells and even less pronounced presence of IGF-1R-positive cells.
Conclusion:
TGF-β1 and HGF are present in defective epithelia and soft tissue in cleft affected lip. Expressions of IGF-1 and IGF-1R show significant differences, and both factors play a role in the morphopathogenesis of clefts.
1. INTRODUCTION
Orofacial clefts - cleft lips, alveolar ridges and palates are one of the most common birth defects with multifactorial and only partly understood morphopathogenesis. Over 500 different complex genetic disorders include cleft formation [1].
TGF-β1 (Transforming growth factor beta 1) belongs to the TGF-β superfamily of approximately 40 cytokines and takes part in controlling proliferation, differentiation and survival in various cell types [2]. TGF-β1 is secreted by myofibroblasts, inflammatory cells, endothelial cells and epithelial cells and takes part in burn scar contracture formation [3]. Similarly, TGF-β1 also modulates wound inflammation and granulation tissue formation [4]. To date, the role of TGF in the formation of clefts has been evaluated in several studies. TGF-β3 is found to be decreasingly expressed in glands and epithelium of unaffected cleft of lip, alveolus with or without cleft palate compared to healthy tissue [5]. Simultaneously, TGF-alpha has been studied in nonsyndromic clefts and healthy control tissue samples; it has been shown to have low protein expression in glands of cleft of the lip or palate tissue, as well as unaffected control tissue obtained from the lip and alveolus [6].
HGF (Hepatocyte growth factor) is secreted by tumor stromal cells; and cancer-associated fibroblasts promote growth, survival and migration of cancer cells in an HGF-dependent manner [7]. HGF is thought to be responsible for cancer cell migration, as well as their invasion via cytoskeletal assembly, reorganization and dynamics. HGF contributes to cytoskeleton remodeling, interactions and distribution [8].
IGF-1 (Insulin like growth factor 1) is phylogenetically ancient neurotrophic hormone with essential role in central nervous system development and maturation. It is especially important in cellular neuroplasticity and signals through its glycoprotein receptor IGF-1R (Insulin like growth factor 1 receptor) [9]. IGF-1 regulates survival and differentiation of many types of cells, including stem cells and placental cells, and stimulates prenatal and postnatal growth. In prenatal life, the synthesis of IGF-1 itself is regulated by various paracrine and endocrine factors and is synthesized as required. IGF-1R is a transmembrane tetramer receptor and its downregulation is associated with the inhibition of differentiation and induction of apoptosis [10]. IGF-1 and IGF-1R also play an initial role in cancerogenesis and their activity leads to increased mitogenesis, cell cycle progression and protection against apoptosis [11].
The aim of this study was to evaluate the presence of TGF-β1, HGF, IGF-1 and IGF-1R in cleft affected mucosa of the lip.
2. MATERIALS AND METHODS
Lip mucosa tissue samples were obtained during surgical lip correction from seven 2 to 6 months old children (five boys and two girls) in the Cleft Lip and Palate Centre at the Institute of Stomatology of Riga Stradins University. Six were patients with unilateral cleft lip and palate and one patient was with bilateral cleft lip and palate.
The study was performed in accordance with the 1964 Declaration of Helsinki. All tissue samples were obtained after receiving written informed consent from the parents of the patients. The study was approved by the Ethical Committee at Riga Stradins University, permit issued in 2003.
Lip mucosa tissue was fixed in Stefanini’s solution [12]. Stefanini’s solution was made of 20 g paraformaldehyde, 150 ml picric acid, 425 ml Sorensen's phosphate buffer (pH 7.2) and 425 ml distilled water, and stored in fridge. Further tissue samples were dehydrated and embedded in paraffin. Four micrometers thick sections were prepared and further stained routinely with hematoxylin and eosin [13].
TGF-β1 (code orb7087, obtained from rabbit, dilution 1:100, Biorbyt Limited, Cambridge, UK), HGF (catalog number AF-294-NA, Lot number ALP01, obtained from goat, dilution 1:300, R&D Systems, USA), IGF-1 (catalog number MAB291, Lot number 56408, obtained from mouse, dilution 1:50, R&D Systems, USA) and IGF-1R (catalog number AF-305-NA, Lot number VL03, obtained from goat, dilution 1:100, R&D Systems, USA) primary antibodies were used by biotin – streptavidin immunohistochemistry (IMH) [14].
Lip mucosa tissue samples were deparaffinized and washed in alcohol and water, then washed for 10 minutes in wash buffer (Tris-buffered saline) and placed for 5 minutes in EDTA boiling buffer in microwave; then cooled down and washed twice for 5 minutes in wash buffer. To decrease background staining, we used normal blocking serum for 20 minutes. All tissue samples were incubated with primary antibodies for 1 hour. Further, washing for 10 minutes in wash buffer and incubation for 30 minutes with LSAB+LINK with biotin related secondary antibodies (code K1015, DakoCytomation, Denmark) was performed. Afterwards we performed another washing for 5 minutes in wash buffer. Lip mucosa samples were incubated for 25 minutes with LSAB+LINK with enzyme peroxidase labeled streptavidine (code K0690, DakoCytomation, Denmark), which was followed by 5 minutes washing in wash buffer and 10 minutes processing with liquid DAB substrate-chromogen system (code K3468, DakoCytomation, Denmark) to obtain positive structure staining in brown color. Samples were then rinsed in running water and counterstained with hematoxylin to stain the nuclei blue.
All antibodies used in the study were tested for positive and negative control.
Image processing and analysis was performed using Image Pro Plus 6.0 software (Media Cybernetics, Silver Spring, Maryland, USA).
The intensity of immunostaining was graded semiquantitatively and labeled as follows: no positive structures in the visual field were labeled as 0, few positive structures in the visual field were labeled with +, moderate number of positive structures in the visual field were labeled with ++, numerous positive structures in the visual field were labeled with +++, abundance of positive structures in the visual field was marked with ++++ [15].
3. RESULTS
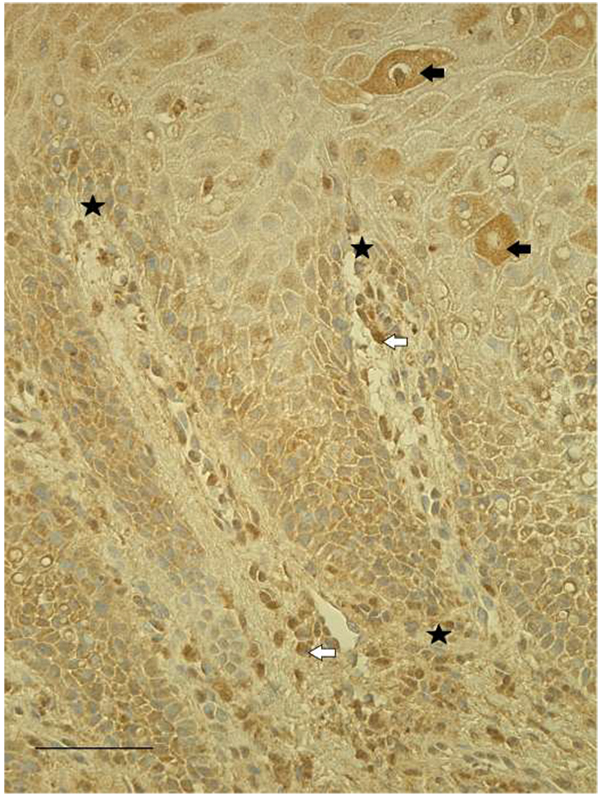
Mostly numerous (+++) TGF-β1-containing structures were detected in all lip tissue samples (Fig. 1). TGF-β1 is found to be expressed in epitheliocytes, as well as fibroblasts and macrophages in subepithelial tissue. In one patient's tissue sample, we observed factor positive salivary glands, while another tissue sample showed patchy immunoreactivity. Occasional endothelial cells of the capillary walls were also immunopositive for TGF-β1.
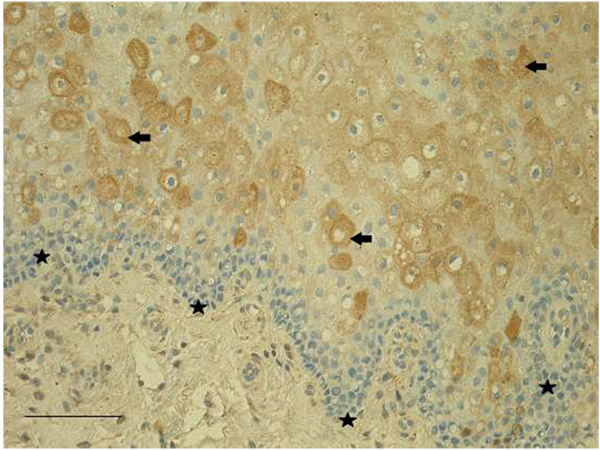
HGF-positive epitheliocytes, macrophages, as well as fibroblasts were found in all patients' tissue samples. In epithelia, we detected mostly numerous (+++) HGF-positive cells, meanwhile HGF-positive fibroblasts and macrophages observed in subepithelial tissue varied from few (+) to abundance (++++) in the visual field (Fig. 2).
Mostly moderate (++) number of IGF-1-containing epithelial cells and connective tissue cells (fibroblasts, macrophages) (Fig. 3), as well as IGF-1R-containing epithelial cells were found in most of our lip mucosa tissue samples. The expression of both factors was often with patchy distribution and the immunoreactive cells were observed in scattered regions. Simultaneously the immunoreactivity of IGF-1R in subepithelial tissue was weak and only some of the tissue samples contained few IGF-1R-positive fibroblasts and macrophages.
4. DISCUSSION
Orofacial clefts are constituted of cleft lip, cleft palate, and cleft lip and palate. Clefts are the largest group of craniofacial malformations. As there is strong familial association, major etiology factor is the genetic component. While cleft palate or cleft clip and palate require surgical correction due to difficulty in breastfeeding, untreated cleft lip or cleft lip and palate leads to social discrimination [16]. Orofacial clefting is associated with delayed facial growth or fusion. Various signaling pathways have been suggested as the culprit for the development of the facial malformations while one detailed mechanism is not known yet [17]. In the current study, we evaluated lip mucosa tissue samples obtained from seven children with cleft lip and palate.
TGF-β1 contributes to the formation of collagenous connective tissue and is expressed by large amount of fibroblasts and chronic inflammatory cells in reactive gingival overgrowths [18]. Compared with typical wounds there is a higher ratio of TGF-β1 in fetal wounds. Fetal wounds are known to heal rapidly and without scar formation early in gestation [19]. Various studies using animal models have looked at the role of TGF in the formation of clefts. It has been found that the loss of TGF-β signaling in the palatal epithelium leads to soft palate muscle cell proliferation and differentiation defects [20]. We found numerous TGF-β1-containing epithelial cells, fibroblasts and macrophages. Some of the endothelial cells in capillaries of our tissue samples were also immunopositive for TGF-β1 and in certain conditions endothelial cells can contain growth factors. We suggest that TGF-β1 takes part in the formation of both defective epithelia and soft tissue in cleft affected lip.
Our tissue samples showed prominent immunoreactivity also for HGF with overall numerous HGF-positive epithelial and subepithelial cells. HGF is associated with head and neck squamous cell carcinoma and contributes to proliferation, metastasis, angiogenesis, the tumor microenvironment and the immune system [21]. Apart from tumorogenesis, it has been shown that HGF is responsible for the development of stria vascularis and nonsensory structures of the cochlea. Lack of HGF signaling in the inner ear leads to hearing loss [22]. HGF is a mitogen and motility factor and primarily regulates epithelial cell function. HGF actively augments the proliferation of corneal epithelial cells in normal and inflammatory conditions [23]. Thus, HGF also takes part in the development of the defective epithelia and soft tissue in cleft affected lip mucosa.
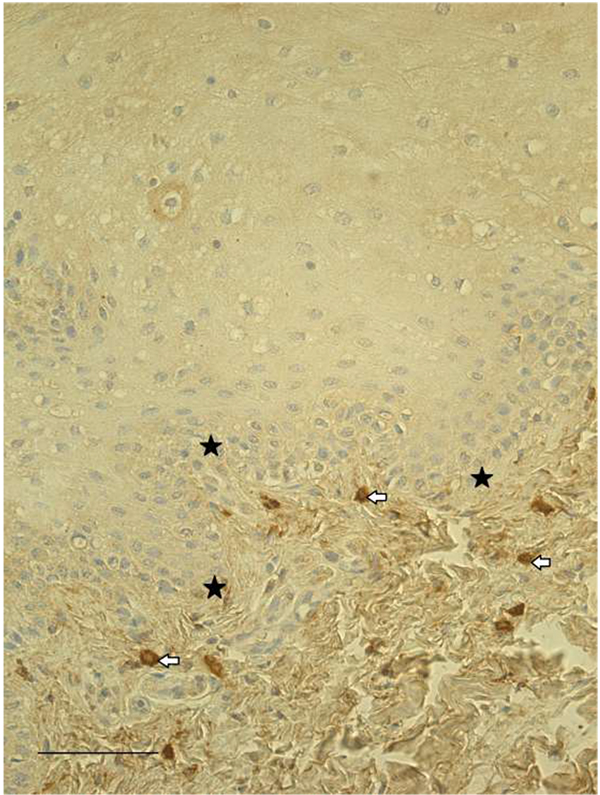
IGF-1 is known to take part in the postnatal facial development as it regulates vertical face growth [24]. IGF-1 also stimulates proliferation, wound healing and differentiation processes of periodontal ligament cells under inflammatory conditions [25]. Furthermore, IGF-1 and IGF-1R are expressed early during embryogenesis and have shown antagonizing effects. Overexpression of IGF-1 leads to anterior expansion of head neural tissue and ectopic eyes, while IGF-1R depletion greatly reduces head structures [26]. We found moderate number of IGF-1-containing epithelial cells and connective tissue cells, as well as IGF-1R-containing epithelial cells. Simultaneously only few IGF-1R-positive connective tissue cells were found in some of the studied tissue samples. Therefore, we suggest that expressions of IGF-1 and IGF-1R show significant differences, and both factors play a role in the morphopathogenesis of clefts.
CONCLUSION
TGF-β1 and HGF are present in defective epithelia and soft tissue in cleft affected lip. Expressions of IGF-1 and IGF-1R show significant differences, and both factors play a role in the morphopathogenesis of clefts.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No Animals/Humans were used for studies that are base of this research.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Riga Stradins University project “Longitudinal research on cleft morphopathogenesis”.


